A systematic review of endpoint definitions in late phase pulmonary tuberculosis therapeutic trials
- PMID: 34344435
- PMCID: PMC8329622
- DOI: 10.1186/s13063-021-05388-1
A systematic review of endpoint definitions in late phase pulmonary tuberculosis therapeutic trials
Abstract
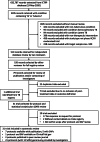
Background: Safe, more efficacious treatments are needed to address the considerable morbidity and mortality associated with pulmonary tuberculosis (TB). However, the current practice in TB therapeutics trials is to use composite binary outcomes, which in the absence of standardization may inflate false positive and negative errors in evaluating regimens. The lack of standardization of outcomes is a barrier to the identification of highly efficacious regimens and the introduction of innovative methodologies METHODS: We conducted a systematic review of trials designed to advance new pulmonary TB drugs or regimens for regulatory approval and inform practice guidelines. Trials were primarily identified from the WHO International Clinical Trial Registry Platform (ICTRP). Only trials that collected post-treatment follow-up data and enrolled at least 100 patients were included. Protocols and Statistical Analysis Plans (SAP) for eligible trials from 1995 to the present were obtained from trial investigators. Details of outcome data, both explicit and implied, were abstracted and organized into three broad categories: favorable, unfavorable, and not assessable. Within these categories, individual trial definitions were recorded and collated, and areas of broad consensus and disagreement were identified and described.
Results: From 2205 trials in any way related to TB, 51 were selected for protocol and SAP review, from which 31 were both eligible and had accessible documentation. Within the three designated categories, we found broad consensus in the definitions of favorable and unfavorable outcomes, although specific details were not always provided, and when explicitly addressed, were heterogeneous. Favorable outcomes were handled the most consistently but were widely variable with respect to specification. In some cases, the same events were defined differently by different protocols, particularly in distinguishing unfavorable from not assessable events. Death was often interpreted as conditional on cause. Patients who did not complete the study because of withdrawal or loss to follow-up presented a particular challenge to consistent interpretation and analytic treatment of outcomes.
Conclusions: In a review of 31 clinical trials, we found that outcome definitions were heterogeneous, highlighting the need to establish clearer specification and a move towards universal standardization of outcomes across pulmonary TB trials. The ICH E9 (R1) addendum provides guidelines for undertaking and achieving this goal.
Prospero registration: PROSPERO CRD42020197993 . Registration 11 August 2020.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- World Health Organization . Global tuberculosis report 2020. Geneva: World Health Organization; 2020.
-
- Mandavilli A. ‘The biggest monster’ is spreading. And it’s not the coronavirus: The New York Times; 2020.
-
- Imperial MZ, Nahid P, Phillips PPJ, Davies GR, Fielding K, Hanna D, Hermann D, Wallis RS, Johnson JL, Lienhardt C, Savic RM. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708–1715. doi: 10.1038/s41591-018-0224-2. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous