Rlf-Mycl Gene Fusion Drives Tumorigenesis and Metastasis in a Mouse Model of Small Cell Lung Cancer
- PMID: 34344693
- PMCID: PMC8810895
- DOI: 10.1158/2159-8290.CD-21-0441
Rlf-Mycl Gene Fusion Drives Tumorigenesis and Metastasis in a Mouse Model of Small Cell Lung Cancer
Abstract
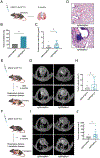
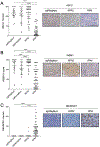
Small cell lung cancer (SCLC) has limited therapeutic options and an exceptionally poor prognosis. Understanding the oncogenic drivers of SCLC may help define novel therapeutic targets. Recurrent genomic rearrangements have been identified in SCLC, most notably an in-frame gene fusion between RLF and MYCL found in up to 7% of the predominant ASCL1-expressing subtype. To explore the role of this fusion in oncogenesis and tumor progression, we used CRISPR/Cas9 somatic editing to generate a Rlf-Mycl-driven mouse model of SCLC. RLF-MYCL fusion accelerated transformation and proliferation of murine SCLC and increased metastatic dissemination and the diversity of metastatic sites. Tumors from the RLF-MYCL genetically engineered mouse model displayed gene expression similarities with human RLF-MYCL SCLC. Together, our studies support RLF-MYCL as the first demonstrated fusion oncogenic driver in SCLC and provide a new preclinical mouse model for the study of this subtype of SCLC.
Significance: The biological and therapeutic implications of gene fusions in SCLC, an aggressive metastatic lung cancer, are unknown. Our study investigates the functional significance of the in-frame RLF-MYCL gene fusion by developing a Rlf-Mycl-driven genetically engineered mouse model and defining the impact on tumor growth and metastasis. This article is highlighted in the In This Issue feature, p. 2945.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest
T.P. has received honoraria/consulting fees from Calithera Biosciences, Vividion Therapeutics and research support from Bristol Myers Squibb, Dracen Pharmaceutical and Agios Pharmaceuticals. C.M.R. has consulted regarding oncology drug development with Amgen, Astra Zeneca, Epizyme, Genentech/Roche, Ipsen, Jazz, Lilly, and Syros. CMR serves on the scientific advisory boards of Bridge Medicines, Earli, and Harpoon Therapeutics.
Figures




References
-
- Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38(21):2369–79 doi 10.1200/JCO.20.00793. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

