Driving regeneration, instead of healing, in adult mammals: the decisive role of resident macrophages through efferocytosis
- PMID: 34344890
- PMCID: PMC8333253
- DOI: 10.1038/s41536-021-00151-1
Driving regeneration, instead of healing, in adult mammals: the decisive role of resident macrophages through efferocytosis
Abstract
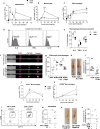
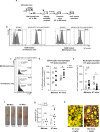
Tissue repair after lesion usually leads to scar healing and thus loss of function in adult mammals. In contrast, other adult vertebrates such as amphibians have the ability to regenerate and restore tissue homeostasis after lesion. Understanding the control of the repair outcome is thus a concerning challenge for regenerative medicine. We recently developed a model of induced tissue regeneration in adult mice allowing the comparison of the early steps of regenerative and scar healing processes. By using studies of gain and loss of function, specific cell depletion approaches, and hematopoietic chimeras we demonstrate here that tissue regeneration in adult mammals depends on an early and transient peak of granulocyte producing reactive oxygen species and an efficient efferocytosis specifically by tissue-resident macrophages. These findings highlight key and early cellular pathways able to drive tissue repair towards regeneration in adult mammals.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

