Delineating the molecular and phenotypic spectrum of the SETD1B-related syndrome
- PMID: 34345025
- PMCID: PMC8553606
- DOI: 10.1038/s41436-021-01246-2
Delineating the molecular and phenotypic spectrum of the SETD1B-related syndrome
Abstract
Purpose: Pathogenic variants in SETD1B have been associated with a syndromic neurodevelopmental disorder including intellectual disability, language delay, and seizures. To date, clinical features have been described for 11 patients with (likely) pathogenic SETD1B sequence variants. This study aims to further delineate the spectrum of the SETD1B-related syndrome based on characterizing an expanded patient cohort.
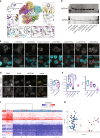
Methods: We perform an in-depth clinical characterization of a cohort of 36 unpublished individuals with SETD1B sequence variants, describing their molecular and phenotypic spectrum. Selected variants were functionally tested using in vitro and genome-wide methylation assays.
Results: Our data present evidence for a loss-of-function mechanism of SETD1B variants, resulting in a core clinical phenotype of global developmental delay, language delay including regression, intellectual disability, autism and other behavioral issues, and variable epilepsy phenotypes. Developmental delay appeared to precede seizure onset, suggesting SETD1B dysfunction impacts physiological neurodevelopment even in the absence of epileptic activity. Males are significantly overrepresented and more severely affected, and we speculate that sex-linked traits could affect susceptibility to penetrance and the clinical spectrum of SETD1B variants.
Conclusion: Insights from this extensive cohort will facilitate the counseling regarding the molecular and phenotypic landscape of newly diagnosed patients with the SETD1B-related syndrome.
© 2021. The Author(s).
Conflict of interest statement
X.W. is employee of Cipher Gene, Ltd. R.E.P., K.G.M., A.C., J.K.R., A.R., and H.Z.E. are employees of GeneDx, Inc. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

