Novel Approaches of Dysregulating Lysosome Functions in Cancer Cells by Specific Drugs and Its Nanoformulations: A Smart Approach of Modern Therapeutics
- PMID: 34345172
- PMCID: PMC8324981
- DOI: 10.2147/IJN.S321343
Novel Approaches of Dysregulating Lysosome Functions in Cancer Cells by Specific Drugs and Its Nanoformulations: A Smart Approach of Modern Therapeutics
Abstract
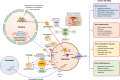
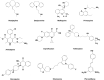
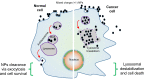
The smart strategy of cancer cells to bypass the caspase-dependent apoptotic pathway has led to the discovery of novel anti-cancer approaches including the targeting of lysosomes. Recent discoveries observed that lysosomes perform far beyond just recycling of cellular waste, as these organelles are metabolically very active and mediate several signalling pathways to sense the cellular metabolic status. These organelles also play a significant role in mediating the immune system functions. Thus, direct or indirect lysosome-targeting with different drugs can be considered a novel therapeutic approach in different disease including cancer. Recently, some anticancer lysosomotropic drugs (eg, nortriptyline, siramesine, desipramine) and their nanoformulations have been engineered to specifically accumulate within these organelles. These drugs can enhance lysosome membrane permeabilization (LMP) or disrupt the activity of resident enzymes and protein complexes, like v-ATPase and mTORC1. Other anticancer drugs like doxorubicin, quinacrine, chloroquine and DQ661 have also been used which act through multi-target points. In addition, autophagy inhibitors, ferroptosis inducers and fluorescent probes have also been used as novel theranostic agents. Several lysosome-specific drug nanoformulations like mixed charge and peptide conjugated gold nanoparticles (AuNPs), Au-ZnO hybrid NPs, TPP-PEG-biotin NPs, octadecyl-rhodamine-B and cationic liposomes, etc. have been synthesized by diverse methods. These nanoformulations can target cathepsins, glucose-regulated protein 78, or other lysosome specific proteins in different cancers. The specific targeting of cancer cell lysosomes with drug nanoformulations is quite recent and faces tremendous challenges like toxicity concerns to normal tissues, which may be resolved in future research. The anticancer applications of these nanoformulations have led them up to various stages of clinical trials. Here in this review article, we present the recent updates about the lysosome ultrastructure, its cross-talk with other organelles, and the novel strategies of targeting this organelle in tumor cells as a recent innovative approach of cancer management.
Keywords: cancer; lysosome; lysosome drug targeting; lysosomotropic agents; nanoparticles.
© 2021 Allemailem et al.
Conflict of interest statement
The authors declare no conflicts of interest for this work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

