Immune Checkpoint Inhibitor-Associated Thrombotic Thrombocytopenic Purpura in a Patient With Metastatic Non-Small-Cell Lung Cancer
- PMID: 34345535
- PMCID: PMC8321598
- DOI: 10.7759/cureus.16035
Immune Checkpoint Inhibitor-Associated Thrombotic Thrombocytopenic Purpura in a Patient With Metastatic Non-Small-Cell Lung Cancer
Abstract
Background: Immune-related adverse events (irAEs) are secondary reactions related to treatment with immune checkpoint inhibitors (ICIs). There have been six cases published reporting on an association between patients undergoing treatment with ICIs and the occurrence of acquired thrombotic thrombocytopenic purpura (TTP).
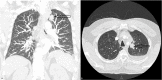
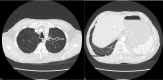
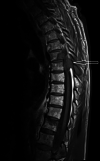
Case report: We report a 61-year-old male receiving treatment with chemoimmunotherapy followed by pembrolizumab maintenance therapy for advanced non-small-cell lung cancer, presenting with bleeding symptoms, anemia, and thrombocytopenia. The patient received pembrolizumab seven times in total, in three-week cycles. Laboratory testing demonstrated hemolytic anemia, which, in combination with other findings, suggested thrombotic microangiopathy (TMA). PLASMIC scoring and specialized testing with ADAMTS13 activity and inhibitor confirmed a diagnosis of TTP. The patient was started on therapy with plasmapheresis and glucocorticoids, resulting in clinical improvement. The patient chose to leave the hospital under the care of home hospice and died approximately one month after being discharged.
Conclusions: Of the six cases of ICI-induced TTP, only one other was treated with pembrolizumab to our knowledge to date. Our patient experienced an adverse reaction marked by thrombocytopenia and hematuria after drug exposure. With symptom improvement after ICI discontinuation and recurrence on readministration, a presumptive diagnosis of ICI-associated TTP was made. This case report and literature review emphasize the need for close observation of patients undergoing ICI therapy for potential rare irAEs. The further investigation aimed at the study of risk factors, disease severity, and treatment response to this form of secondary TTP is needed to guide treatment decisions.
Keywords: immune checkpoint inhibitors; immune related adverse events; pembrolizumab; thrombotic microangiopathy; thrombotic thrombocytopenic purpura.
Copyright © 2021, De Filippis et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
A Rare But Fatal Toxicity: Immune Checkpoint Inhibitor-Related Acquired Thrombotic Thrombocytopenic Purpura.J Immunother Precis Oncol. 2025 Jan 10;8(1):15-22. doi: 10.36401/JIPO-24-2. eCollection 2025 Feb. J Immunother Precis Oncol. 2025. PMID: 39811422 Free PMC article. Review.
-
Thrombotic microangiopathy in malignant hypertension and hemolytic uremic syndrome (HUS)/ thrombotic thrombocytopenic purpura (TTP): can we differentiate one from the other?Hypertens Res. 2005 Jan;28(1):89-95. doi: 10.1291/hypres.28.89. Hypertens Res. 2005. PMID: 15969259
-
Pembrolizumab-induced thrombotic thrombocytopenic purpura.J Oncol Pharm Pract. 2020 Jul;26(5):1237-1240. doi: 10.1177/1078155219887212. Epub 2019 Nov 13. J Oncol Pharm Pract. 2020. PMID: 31718453
-
Recent advances in thrombotic thrombocytopenic purpura.Hematology Am Soc Hematol Educ Program. 2004:407-23. doi: 10.1182/asheducation-2004.1.407. Hematology Am Soc Hematol Educ Program. 2004. PMID: 15561695 Review.
-
Thrombotic Thrombocytopenic Purpura Induced by Immune Checkpoint Inhibitiors: A Case Report and Review of the Literature.Cureus. 2020 Oct 29;12(10):e11246. doi: 10.7759/cureus.11246. Cureus. 2020. PMID: 33274128 Free PMC article.
Cited by
-
Rare Immune-Related Adverse Events (irAEs): Approach to Diagnosis and Management.Pharmaceut Med. 2024 Jan;38(1):25-38. doi: 10.1007/s40290-023-00508-5. Epub 2024 Jan 9. Pharmaceut Med. 2024. PMID: 38194017 Free PMC article. Review.
-
Renal-Limited Thrombotic Microangiopathy in a Patient Who Received Gemcitabine, Ramucirumab, and Pembrolizumab: A Case Report and Literature Review.Cureus. 2024 Feb 5;16(2):e53669. doi: 10.7759/cureus.53669. eCollection 2024 Feb. Cureus. 2024. PMID: 38455838 Free PMC article.
-
Nivolumab-induced Thrombotic Thrombocytopenic Purpura in Patients with Gastric Tube Cancer.Intern Med. 2024 Oct 1;63(19):2667-2671. doi: 10.2169/internalmedicine.2931-23. Epub 2024 Mar 4. Intern Med. 2024. PMID: 38432967 Free PMC article.
-
Assessing hemorrhagic risks in combination therapy: implications of angiogenesis inhibitors and immune checkpoint inhibitors.Front Immunol. 2025 Feb 10;16:1527570. doi: 10.3389/fimmu.2025.1527570. eCollection 2025. Front Immunol. 2025. PMID: 39995676 Free PMC article.
-
Plasma exchange for severe immune-related adverse events from checkpoint inhibitors: an early window of opportunity?Immunother Adv. 2022 May 27;2(1):ltac012. doi: 10.1093/immadv/ltac012. eCollection 2022. Immunother Adv. 2022. PMID: 35814850 Free PMC article. Review.
References
-
- Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Anagnostou VK, Brahmer JR. Clin Cancer Res. 2015;21:976–984. - PubMed
-
- Pembrolizumab-induced thrombotic thrombocytopenic purpura. Dickey MS, Raina AJ, Gilbar PJ, Wisniowski BL, Collins JT, Karki B, Nguyen AD. J Oncol Pharm Pract. 2020;26:1237–1240. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
