Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients
- PMID: 34346068
- PMCID: PMC8444745
- DOI: 10.1111/bjh.17743
Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients
Abstract
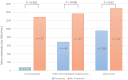
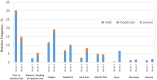
Haemato-oncological patients are at risk in case of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Currently, vaccination is the best-evaluated preventive strategy. In the present study, we aimed to assess serological response, predictive markers, and safety of BNT162b2 in haemato-oncological patients. A total of 259 haemato-oncological patients were vaccinated with two 30 µg doses of BNT162b2 administered 21 days apart. Serological response was assessed by ELECSYS® Anti-SARS-CoV-2-S immunoassay before vaccination, and at 3 and 7 weeks after the first dose (T1, T2). Safety assessment was performed. At T2 spike protein receptor binding domain (S/RBD) antibodies were detected in 71·4% of haematological and in 94·5% of oncological patients (P < 0·001). Haematological patients receiving systemic treatment had a 14·2-fold increased risk of non-responding (95% confidence interval 3·2-63·3, P = 0·001). Subgroups of patients with lymphoma or chronic lymphocytic leukaemia were at highest risk of serological non-response. Low immunoglobulin G (IgG) level, lymphocyte- and natural killer (NK)-cell counts were significantly associated with poor serological response (P < 0·05). Vaccination was well tolerated with only 2·7% of patients reporting severe side-effects. Patients with side-effects developed a higher S/RBD-antibody titre compared to patients without side-effects (P = 0·038). Haematological patients under treatment were at highest risk of serological non-response. Low lymphocytes, NK cells and IgG levels were found to be associated with serological non-response. Serological response in oncological patients was encouraging. The use of BNT162b2 is safe in haemato-oncological patients.
Keywords: BNT162b2 mRNA vaccine; COVID-19; chronic lymphocytic leukaemia; immune cells; serological response.
© 2021 British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflict of interests with the present study.
Figures



References
-
- Johns Hopkins University Coronavirus Resource Center . COVID‐19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University; 2020. Available from: https://coronavirus.jhu.edu/map.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

