Rolling controls sperm navigation in response to the dynamic rheological properties of the environment
- PMID: 34346314
- PMCID: PMC8387022
- DOI: 10.7554/eLife.68693
Rolling controls sperm navigation in response to the dynamic rheological properties of the environment
Abstract
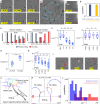
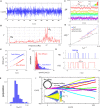
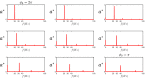
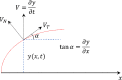
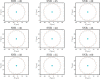
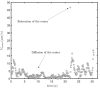
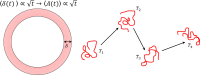
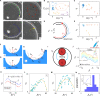
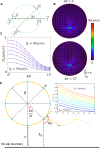
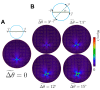
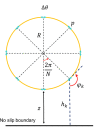
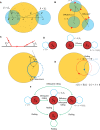
Mammalian sperm rolling around their longitudinal axes is a long-observed component of motility, but its function in the fertilization process, and more specifically in sperm migration within the female reproductive tract, remains elusive. While investigating bovine sperm motion under simple shear flow and in a quiescent microfluidic reservoir and developing theoretical and computational models, we found that rolling regulates sperm navigation in response to the rheological properties of the sperm environment. In other words, rolling enables a sperm to swim progressively even if the flagellum beats asymmetrically. Therefore, a rolling sperm swims stably along the nearby walls (wall-dependent navigation) and efficiently upstream under an external fluid flow (rheotaxis). By contrast, an increase in ambient viscosity and viscoelasticity suppresses rolling, consequently, non-rolling sperm are less susceptible to nearby walls and external fluid flow and swim in two-dimensional diffusive circular paths (surface exploration). This surface exploration mode of swimming is caused by the intrinsic asymmetry in flagellar beating such that the curvature of a sperm's circular path is proportional to the level of asymmetry. We found that the suppression of rolling is reversible and occurs in sperm with lower asymmetry in their beating pattern at higher ambient viscosity and viscoelasticity. Consequently, the rolling component of motility may function as a regulatory tool allowing sperm to navigate according to the rheological properties of the functional region within the female reproductive tract.
Keywords: ambient rheology; b. taurus; developmental biology; female reproductive tract; navigation; physics of living systems; sperm rolling; surface exploration.
© 2021, Zaferani et al.
Conflict of interest statement
MZ, FJ, AM, PL, AA No competing interests declared
Figures






References
-
- Bénichou O, Loverdo C, Moreau M, Voituriez R. Intermittent search strategies. Reviews of Modern Physics. 2011;83:81–129. doi: 10.1103/RevModPhys.83.81. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

