Single-molecule fluorescence vistas of how lipids regulate membrane proteins
- PMID: 34346484
- PMCID: PMC8848334
- DOI: 10.1042/BST20201074
Single-molecule fluorescence vistas of how lipids regulate membrane proteins
Abstract
The study of membrane proteins is undergoing a golden era, and we are gaining unprecedented knowledge on how this key group of proteins works. However, we still have only a basic understanding of how the chemical composition and the physical properties of lipid bilayers control the activity of membrane proteins. Single-molecule (SM) fluorescence methods can resolve sample heterogeneity, allowing to discriminate between the different molecular populations that biological systems often adopt. This short review highlights relevant examples of how SM fluorescence methodologies can illuminate the different ways in which lipids regulate the activity of membrane proteins. These studies are not limited to lipid molecules acting as ligands, but also consider how the physical properties of the bilayer can be determining factors on how membrane proteins function.
Keywords: EphA2; PIP2; SMALP; cholesterol; fluorescence resonance energy transfer; hydrophobic mismatch.
© 2021 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures

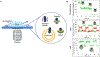
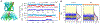
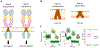

References
-
- van MG, de Kroon AI. Lipid map of the mammalian cell. J Cell Sci. 2011;124(Pt 1):5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

