Evolutionary and Functional Analysis of Coagulase Positivity among the Staphylococci
- PMID: 34346700
- PMCID: PMC8386474
- DOI: 10.1128/mSphere.00381-21
Evolutionary and Functional Analysis of Coagulase Positivity among the Staphylococci
Abstract
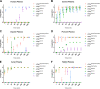
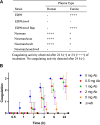
The bacterial genus Staphylococcus comprises a large group of pathogenic and nonpathogenic species associated with an array of host species. Staphylococci are differentiated into coagulase-positive or coagulase-negative groups based on the capacity to promote clotting of plasma, a phenotype historically associated with the ability to cause disease. However, the genetic basis of this important diagnostic and pathogenic trait across the genus has not been examined to date. Here, we selected 54 representative staphylococcal species and subspecies to examine coagulation of plasma derived from six representative host species. In total, 13 staphylococcal species mediated coagulation of plasma from at least one host species including one previously identified as coagulase negative (Staphylococcus condimenti). Comparative genomic analysis revealed that coagulase activity correlated with the presence of a gene (vwb) encoding the von Willebrand binding protein (vWbp) whereas only the Staphylococcus aureus complex contained a gene encoding staphylocoagulase (Coa), the classical mediator of coagulation. Importantly, S. aureus retained vwb-dependent coagulase activity in an S. aureus strain deleted for coa whereas deletion of vwb in Staphylococcus pseudintermedius resulted in loss of coagulase activity. Whole-genome-based phylogenetic reconstruction of the Staphylococcus genus revealed that the vwb gene has been acquired on at least four different occasions during the evolution of the Staphylococcus genus followed by allelic diversification via mutation and recombination. Allelic variants of vWbp from selected coagulase-positive staphylococci mediated coagulation in a host-dependent manner indicative of host-adaptive evolution. Taken together, we have determined the genetic and evolutionary basis of staphylococcal coagulation, revealing vWbp to be its archetypal determinant. IMPORTANCE The ability of some species of staphylococci to promote coagulation of plasma is a key pathogenic and diagnostic trait. Here, we provide a comprehensive analysis of the coagulase positivity of the staphylococci and its evolutionary genetic basis. We demonstrate that the von Willebrand binding protein rather than staphylocoagulase is the archetypal coagulation factor of the staphylococci and that the vwb gene has been acquired several times independently during the evolution of the staphylococci. Subsequently, vwb has undergone adaptive diversification to facilitate host-specific functionality. Our findings provide important insights into the evolution of pathogenicity among the staphylococci and the genetic basis for a defining diagnostic phenotype.
Keywords: Staphylococcus; coagulase; coagulase-positive staphylococci; coagulation; diagnostics; evolution; host specificity; phylogeny; von Willebrand binding protein.
Figures
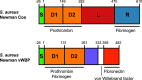
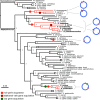
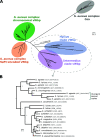
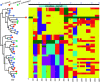
References
-
- Smith W, Hale JH. 1944. The nature and mode of action of Staphylococcus coagulase. Br J Exp Pathol 25:101–110.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

