Analysis of Cryptococcal Extracellular Vesicles: Experimental Approaches for Studying Their Diversity Among Multiple Isolates, Kinetics of Production, Methods of Separation, and Detection in Cultures of Titan Cells
- PMID: 34346749
- PMCID: PMC8552642
- DOI: 10.1128/Spectrum.00125-21
Analysis of Cryptococcal Extracellular Vesicles: Experimental Approaches for Studying Their Diversity Among Multiple Isolates, Kinetics of Production, Methods of Separation, and Detection in Cultures of Titan Cells
Abstract
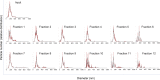
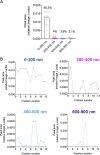
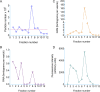
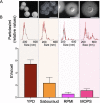
Extracellular vesicles (EVs) produced by members of the Cryptococcus genus are associated with fundamental processes of fungal physiology and virulence. However, several questions about the properties of cryptococcal EVs remain unanswered, mostly because of technical limitations. We recently described a fast and efficient protocol of high-yield EV isolation from solid medium. In this study, we aimed at using the solid medium protocol to address some of the open questions about EVs, including the kinetics of EV production, the diversity of EVs produced by multiple isolates under different culture conditions, the separation of vesicles in a density gradient followed by the recovery of functional EVs, the direct detection of EVs in culture supernatants, and the production of vesicles in solid cultures of Titan cells. Our results indicate that the production of EVs is directly impacted by the culture medium and time of growth, resulting in variable detection of EVs per cell and a peak of EV detection at 24 h of growth. Nanoparticle tracking analysis (NTA) of EV samples revealed that multiple isolates produce vesicles with variable properties, including particles of diverging dimensions. EVs were produced in the solid medium in amounts that were separated on a centrifugation density gradient, resulting in the recovery of functional EVs containing the major cryptococcal capsular antigen. We also optimized the solid medium protocol for induction of the formation of Titan cells, and analyzed the production of EVs by NTA and transmission electron microscopy. This analysis confirmed that EVs were isolated from solid cultures of cryptococcal enlarged cells. With these approaches, we expect to implement simple methods that will facilitate the analysis of EVs produced by fungal cells. IMPORTANCE Fungal extracellular vesicles (EVs) are considered to be important players in the biology of fungal pathogens. However, the limitations in the methodological approaches to studying fungal EVs impair the expansion of knowledge in this field. In the present study, we used the Cryptococcus genus as a model for the study of EVs. We explored the simplification of protocols for EV analysis, which helped us to address some important, but still unanswered, questions about fungal EVs.
Keywords: Cryptococcus; extracellular vesicles; pathogenesis.
Figures



References
-
- Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59. doi: 10.1128/EC.00318-06. - DOI - PMC - PubMed
-
- Reis F, Borges BS, Jozefowicz L, Sena B, Garcia A, Medeiros LC, Martins S, Honorato L, Schrank A, Vainstein MHMH, Kmetzsch L, Nimrichter L, Alves L, Staats CCCC, Rodrigues MLML. 2019. A novel protocol for the isolation of fungal extracellular vesicles reveals the participation of a putative scramblase in polysaccharide export and capsule construction in Cryptococcus gattii. mSphere 4:e00080-19. doi: 10.1128/mSphere.00080-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

