Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects
- PMID: 34346750
- PMCID: PMC8552678
- DOI: 10.1128/Spectrum.00341-21
Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects
Abstract
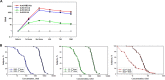
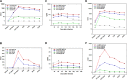
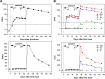
Knowledge about development and duration of virus-specific antibodies after COVID-19 vaccination is important for understanding how to limit the pandemic via vaccination in different populations and societies. However, the clinical utility of postvaccination testing of antibody response and selection of targeted SARS-CoV-2 antigen(s) has not been established. The results of such testing from clinical teams independent from vaccine manufacturers are also limited. Here, we report the initial results of an ongoing clinical study on evaluation of antibody response to four different SARS-CoV-2 antigens after first and second dose of Pfizer and Moderna mRNA vaccines and at later time points. We revealed a peak of antibody induction after the vaccine boosting dose with a gradual decline of antibody levels at later time. Anti-nucleocapsid antibody was not induced by spike protein-encoding vaccines and this may continue to serve as a marker of previous SARS-CoV-2 infection. No differences between the two vaccines in terms of antibody response were revealed. Age and gender dependencies were determined to be minimal within the healthy adult (but not aged) population. Our results suggest that postvaccination testing of antibody response is an important and feasible tool for following people after vaccination and selecting individuals who might require a third dose of vaccine at an earlier time point or persons who may not need a second dose due to previous SARS-CoV-2 infection. IMPORTANCE Now that authorized vaccines for COVID-19 have been widely used, it is important to understand how they induce antivirus antibodies, which antigens are targeted, how long antibodies circulate, and how personal health conditions and age may affect this humoral immunity. Here, we report induction and time course of multiple anti-SARS-CoV-2 antibody responses in healthy individuals immunized with Pfizer and Moderna mRNA vaccines. We also determined the age and gender dependence of the antibody response and compared antibody levels to responses seen in those who have recovered from COVID-19. Our results suggest the importance of screening for antibody response to multiple antigens after vaccination in order to reveal individuals who require early and late additional boosting and those who may not need second dose due to prior SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; antibody; humoral immunity; vaccination.
Figures




References
-
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. 2021. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371:eabf4063. doi: 10.1126/science.abf4063. - DOI - PMC - PubMed
-
- Food and Drug Administration. 2020. Pfizer COVID-19 vaccine emergency use authorization. U.S. Food and Drug Administration, Silver Spring, MD.
-
- Food and Drug Administration. 2020. Moderna COVID-19 vaccine emergency use authorization. U.S. Food and Drug Administration, Silver Spring, MD.
-
- Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, Malyutin AG, Sharaf NG, Huey-Tubman KE, Lee YE, Robbiani DF, Nussenzweig MC, West AP, Jr, Bjorkman PJ. 2020. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588:682–687. doi: 10.1038/s41586-020-2852-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

