Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody
- PMID: 34346827
- PMCID: PMC8382006
- DOI: 10.1080/22221751.2021.1960900
Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody
Abstract
MW33 is a fully humanized IgG1κ monoclonal neutralizing antibody, and may be used for the prevention and treatment of coronavirus disease 2019 (COVID-19). We conducted a randomized, double-blind, placebo-controlled, single-dose, dose-escalation Phase 1 study to evaluate the safety, tolerability, pharmacokinetics (PK), and immunogenicity of MW33. Healthy adults aged 18-45 years were sequentially enrolled into the 4, 10, 20, 40, and 60 mg/kg dose groups and infused with MW33 over 60 ± 15 min and followed for 85 days. All 42 enrolled participants completed the MW33 infusion, and 40 participants completed the 85-day follow-up period. 34 participants received a single infusion of 4 (n = 2), 10 (n = 8), 20 (n = 8), 40 (n = 8), and 60 mg/kg (n = 8) of MW33. 27 subjects in the test groups experienced 78 adverse events (AEs) post-dose, with an incidence of 79.4% (27/34). The most common AEs included abnormal laboratory test results, vascular and lymphatic disorders, and infectious diseases. The severity of AEs was mainly Grade 1 (92 AEs), and three Grade 2 and one Grade 4. The main PK parameters, maximum concentration (Cmax), and area under the concentration-time curve (AUC0-t, and AUC0-∞) in 34 subjects showed a linear kinetic relationship in the range of 10-60 mg/kg. The plasma half-life was approximately 25 days. The positive rates of serum ADAs and antibody titres were low with no evidence of an impact on safety or PK. In conclusion, MW33 was well-tolerated, demonstrated linear PK, with a lower positive rate of serum ADAs and antibody titres in healthy subjects.Trial registration: ClinicalTrials.gov identifier: NCT04427501.Trial registration: ClinicalTrials.gov identifier: NCT04533048.Trial registration: ClinicalTrials.gov identifier: NCT04627584.
Keywords: COVID-19; MW33 injection; Phase 1 clinical trial; SARS-CoV-2; monoclonal antibody; pharmacokinetic characteristics; safety.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

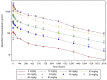
Similar articles
-
Safety, tolerability, pharmacokinetics, and immunogenicity of an anti-SARS-CoV-2 monoclonal antibody HFB30132A after single dose intravenous administration in healthy Chinese subjects: a phase 1, randomized, double-blind, placebo-controlled study.Front Pharmacol. 2023 May 23;14:1117293. doi: 10.3389/fphar.2023.1117293. eCollection 2023. Front Pharmacol. 2023. PMID: 37332355 Free PMC article.
-
Safety, Tolerability, Pharmacokinetics, and Immunogenicity of a Monoclonal Antibody (SCTA01) Targeting SARS-CoV-2 in Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled, Phase I Study.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0106321. doi: 10.1128/AAC.01063-21. Epub 2021 Sep 7. Antimicrob Agents Chemother. 2021. PMID: 34491805 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Inflammation, immunity and potential target therapy of SARS-COV-2: A total scale analysis review.Food Chem Toxicol. 2021 Apr;150:112087. doi: 10.1016/j.fct.2021.112087. Epub 2021 Feb 25. Food Chem Toxicol. 2021. PMID: 33640537 Free PMC article. Review.
-
When Therapeutic IgA Antibodies Might Come of Age.Pharmacology. 2021;106(1-2):9-19. doi: 10.1159/000510251. Epub 2020 Sep 18. Pharmacology. 2021. PMID: 32950975 Review.
Cited by
-
Therapeutic antibodies for COVID-19: is a new age of IgM, IgA and bispecific antibodies coming?MAbs. 2022 Jan-Dec;14(1):2031483. doi: 10.1080/19420862.2022.2031483. MAbs. 2022. PMID: 35220888 Free PMC article. Review.
-
Research and development of Chinese anti-COVID-19 drugs.Acta Pharm Sin B. 2022 Dec;12(12):4271-4286. doi: 10.1016/j.apsb.2022.09.002. Epub 2022 Sep 13. Acta Pharm Sin B. 2022. PMID: 36119967 Free PMC article. Review.
-
First-in-Human Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a Rapidly Developed SARS-CoV-2 Therapeutic Antibody, AOD01, in Healthy Adults.Infect Dis Ther. 2022 Oct;11(5):1999-2015. doi: 10.1007/s40121-022-00681-1. Epub 2022 Sep 4. Infect Dis Ther. 2022. PMID: 36058990 Free PMC article.
-
Safety, Pharmacokinetics, Serum Neutralizing Titers, and Immunogenicity of Adintrevimab, a Monoclonal Antibody Targeting SARS-CoV-2: A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Dose-escalation Study in Healthy Adults.Infect Dis Ther. 2023 May;12(5):1365-1377. doi: 10.1007/s40121-023-00794-1. Epub 2023 Apr 25. Infect Dis Ther. 2023. PMID: 37185797 Free PMC article.
-
Antigenicity comparison of SARS-CoV-2 Omicron sublineages with other variants contained multiple mutations in RBD.MedComm (2020). 2022 Apr 9;3(2):e130. doi: 10.1002/mco2.130. eCollection 2022 Jun. MedComm (2020). 2022. PMID: 35434713 Free PMC article.
References
-
- Sakiko Tabata KISK, Tsutomu Kodera MKMS.. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier connect, the company's public news and information website. Lancet Inf Dis. 2020;20:1043–1050.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
