Eosinophils are part of the granulocyte response in tuberculosis and promote host resistance in mice
- PMID: 34347010
- PMCID: PMC8348215
- DOI: 10.1084/jem.20210469
Eosinophils are part of the granulocyte response in tuberculosis and promote host resistance in mice
Abstract
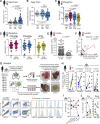
Host resistance to Mycobacterium tuberculosis (Mtb) infection requires the activities of multiple leukocyte subsets, yet the roles of the different innate effector cells during tuberculosis are incompletely understood. Here we uncover an unexpected association between eosinophils and Mtb infection. In humans, eosinophils are decreased in the blood but enriched in resected human tuberculosis lung lesions and autopsy granulomas. An influx of eosinophils is also evident in infected zebrafish, mice, and nonhuman primate granulomas, where they are functionally activated and degranulate. Importantly, using complementary genetic models of eosinophil deficiency, we demonstrate that in mice, eosinophils are required for optimal pulmonary bacterial control and host survival after Mtb infection. Collectively, our findings uncover an unexpected recruitment of eosinophils to the infected lung tissue and a protective role for these cells in the control of Mtb infection in mice.
© 2021 Bohrer et al.
Conflict of interest statement
Disclosures: D.M. Lowe reported personal fees from Merck, non-financial support from CSL Behring, and non-financial support from Fujifilm Chemical Co. outside the submitted work. No other disclosures were reported.
Figures







References
-
- Abate, E., Belayneh M., Idh J., Diro E., Elias D., Britton S., Aseffa A., Stendahl O., and Schön T.. 2015. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLoS Negl. Trop. Dis. 9:e0003994. 10.1371/journal.pntd.0003994 - DOI - PMC - PubMed
-
- Arnold, I.C., Artola-Borán M., Tallón de Lara P., Kyburz A., Taube C., Ottemann K., van den Broek M., Yousefi S., Simon H.U., and Müller A.. 2018. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 215:2055–2072. 10.1084/jem.20172049 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

