Netupitant/palonosetron without dexamethasone for preventing nausea and vomiting in patients with multiple myeloma receiving high-dose melphalan for autologous stem cell transplantation: a single-center experience
- PMID: 34347181
- PMCID: PMC8331991
- DOI: 10.1007/s00520-021-06472-7
Netupitant/palonosetron without dexamethasone for preventing nausea and vomiting in patients with multiple myeloma receiving high-dose melphalan for autologous stem cell transplantation: a single-center experience
Abstract
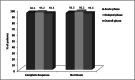
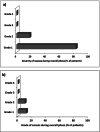
Chemotherapy-induced nausea and vomiting (CINV) is one of the most frequent adverse events compromising quality of life (QoL) in patients undergoing autologous stem cell transplantation (ASCT). However, CINV prophylaxis is still lacking uniformity for high-dose melphalan (HDM), which is used to condition patients with multiple myeloma (MM). Netupitant/palonosetron (NEPA) is administered with dexamethasone (DEXA) for CINV prevention in several chemotherapy regimens. Our study aims to assess the efficacy of NEPA, without DEXA, in preventing CINV in 106 adult patients with MM receiving HDM and ASCT. All patients had antiemetic prophylaxis with multiple doses of NEPA 1 h before the start of conditioning and after 72 h and 120 h. A complete response (CR) was observed in 99 (93%) patients at 120 h (overall phase). The percentage of patients with complete control was 93%. The CR rate during the acute phase was 94% (n = 100). During the delayed phase, the CR rate was 95% (n = 101). Grade 1 nausea and vomiting were experienced by 82% and 12% of the patients, respectively. Grade 2 nausea was reported in 18% and vomiting in 10% of patients. Our results showed, for the first time, that NEPA, without DEXA, was a well-tolerated and effective antiemetic option for MM patients receiving HDM followed by ASCT.
Keywords: ASCT; Chemotherapy-induced nausea and vomiting (CINV); High-dose melphalan; Multiple myeloma; Netupitant/palonosetron (NEPA).
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
ADC is an employee of Italfarmaco SpA. Other authors have no conflict of interest to disclose.
Figures
References
-
- Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, Sarosiek T, Oprean C, Cardona-Huerta S, Lorusso V, Karthaus M, Schwartzberg L, Grunberg S. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1328–1333. doi: 10.1093/annonc/mdu101. - DOI - PMC - PubMed
-
- Apolito V, Giaccone L, Ferrero S, Larocca A, Cavallo F, Coscia M, Beggiato E, Butera S, Martella F, Dainese C, Cetani G, Scaldaferri M, Cattel F, Boccadoro M, Ferrero D, Bruno B, Cerrano M. Netupitant-palonosetron to prevent chemotherapy-induced nausea and vomiting in multiple myeloma patients receiving high-dose melphalan and autologous stem cell transplantation. Ann Hematol. 2020;99:2197–2199. doi: 10.1007/s00277-020-04180-6. - DOI - PubMed
-
- Bechtel T, McBride A, Crawford B, Bullington S, Hofmeister CC, Benson DM, Jr, Jaglowski S, Penza S, Andritsos LA, Devine SM. Aprepitant for the control of delayed nausea and vomiting associated with the use of high-dose melphalan for autologous peripheral blood stem cell transplants in patients with multiple myeloma: a phase II study. Support Care Cancer. 2014;22:2911–2916. doi: 10.1007/s00520-014-2248-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

