Adaptive Synthesis of a Rough Lipopolysaccharide in Geobacter sulfurreducens for Metal Reduction and Detoxification
- PMID: 34347518
- PMCID: PMC8478458
- DOI: 10.1128/AEM.00964-21
Adaptive Synthesis of a Rough Lipopolysaccharide in Geobacter sulfurreducens for Metal Reduction and Detoxification
Abstract
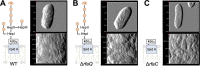
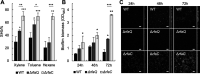
The ability of some metal-reducing bacteria to produce a rough (no O-antigen) lipopolysaccharide (LPS) could facilitate surface interactions with minerals and metal reduction. Consistent with this, the laboratory model metal reducer Geobacter sulfurreducens PCA produced two rough LPS isoforms (with or without a terminal methyl-quinovosamine sugar) when growing with the soluble electron acceptor fumarate but expressed only the shorter and more hydrophilic variant when reducing iron oxides. We reconstructed from genomic data conserved pathways for the synthesis of the rough LPS and generated heptosyltransferase mutants with partial (ΔrfaQ) or complete (ΔrfaC) truncations in the core oligosaccharide. The stepwise removal of the LPS core sugars reduced the hydrophilicity of the cell and increased outer membrane vesiculation. These changes in surface charge and remodeling did not substantially impact planktonic growth but disrupted the developmental stages and structure of electroactive biofilms. Furthermore, the mutants assembled conductive pili for extracellular mineralization of the toxic uranyl cation but were unable to prevent permeation and mineralization of the radionuclide in the cell envelope. Hence, not only does the rough LPS promote cell-cell and cell-mineral interactions critical to biofilm formation and metal respiration but it also functions as a permeability barrier to toxic metal cations. In doing so, the rough LPS maximizes the extracellular reduction of soluble and insoluble metals and preserves cell envelope functions critical to the environmental survival of Geobacter bacteria in metal-rich environments and their performance in bioremediation and bioenergy applications. IMPORTANCE Some metal-reducing bacteria produce an LPS without the repeating sugars (O-antigen) that decorate the surface of most Gram-negative bacteria, but the biological significance of this adaptive feature was not previously investigated. Using the model representative Geobacter sulfurreducens strain PCA and mutants carrying stepwise truncations in the LPS core sugars, we demonstrate the importance of the rough LPS in the control of cell surface chemistry during the respiration of iron minerals and the formation of electroactive biofilms. Importantly, we describe hitherto overlooked roles for the rough LPS in metal sequestration and outer membrane vesiculation that are critical for the extracellular reduction and detoxification of toxic metals and radionuclides. These results are of interest for the optimization of bioremediation schemes and electricity-harvesting platforms using these bacteria.
Keywords: biofilms; cell envelope; electromicrobiology; extracellular electron transfer; metal reduction; outer membrane vesicles; type IV pili.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

