Lapatinib and poziotinib overcome ABCB1-mediated paclitaxel resistance in ovarian cancer
- PMID: 34347777
- PMCID: PMC8336885
- DOI: 10.1371/journal.pone.0254205
Lapatinib and poziotinib overcome ABCB1-mediated paclitaxel resistance in ovarian cancer
Abstract
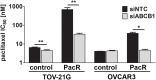
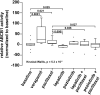
Conventional frontline treatment for ovarian cancer consists of successive chemotherapy cycles of paclitaxel and platinum. Despite the initial favorable responses for most patients, chemotherapy resistance frequently leads to recurrent or refractory disease. New treatment strategies that circumvent or prevent mechanisms of resistance are needed to improve ovarian cancer therapy. We established in vitro paclitaxel-resistant ovarian cancer cell line and organoid models. Gene expression differences in resistant and sensitive lines were analyzed by RNA sequencing. We manipulated candidate genes associated with paclitaxel resistance using siRNA or small molecule inhibitors, and then screened the cells for paclitaxel sensitivity using cell viability assays. We used the Bliss independence model to evaluate the anti-proliferative synergy for drug combinations. ABCB1 expression was upregulated in paclitaxel-resistant TOV-21G (q < 1x10-300), OVCAR3 (q = 7.4x10-156) and novel ovarian tumor organoid (p = 2.4x10-4) models. Previous reports have shown some tyrosine kinase inhibitors can inhibit ABCB1 function. We tested a panel of tyrosine kinase inhibitors for the ability to sensitize resistant ABCB1-overexpressing ovarian cancer cell lines to paclitaxel. We observed synergy when we combined poziotinib or lapatinib with paclitaxel in resistant TOV-21G and OVCAR3 cells. Silencing ABCB1 expression in paclitaxel-resistant TOV-21G and OVCAR3 cells reduced paclitaxel IC50 by 20.7 and 6.2-fold, respectively. Furthermore, we demonstrated direct inhibition of paclitaxel-induced ABCB1 transporter activity by both lapatinib and poziotinib. In conclusion, lapatinib and poziotinib combined with paclitaxel synergizes to inhibit the proliferation of ABCB1-overexpressing ovarian cancer cells in vitro. The addition of FDA-approved lapatinib to second-line paclitaxel therapy is a promising strategy for patients with recurrent ovarian cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



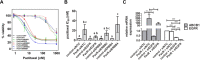
Similar articles
-
Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells.Mol Cancer Ther. 2004 Jul;3(7):833-8. Mol Cancer Ther. 2004. PMID: 15252144
-
ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells.Br J Cancer. 2016 Aug 9;115(4):431-41. doi: 10.1038/bjc.2016.203. Epub 2016 Jul 14. Br J Cancer. 2016. PMID: 27415012 Free PMC article.
-
Alkaliptosis induction counteracts paclitaxel-resistant ovarian cancer cells via ATP6V0D1-mediated ABCB1 inhibition.Mol Carcinog. 2024 Aug;63(8):1515-1527. doi: 10.1002/mc.23741. Epub 2024 May 15. Mol Carcinog. 2024. PMID: 38751020
-
The Syk inhibitor R406 is a modulator of P-glycoprotein (ABCB1)-mediated multidrug resistance.PLoS One. 2019 Jan 22;14(1):e0210879. doi: 10.1371/journal.pone.0210879. eCollection 2019. PLoS One. 2019. PMID: 30668583 Free PMC article.
-
Key genes and molecular mechanisms related to Paclitaxel Resistance.Cancer Cell Int. 2024 Jul 13;24(1):244. doi: 10.1186/s12935-024-03415-0. Cancer Cell Int. 2024. PMID: 39003454 Free PMC article. Review.
Cited by
-
Combined inhibition of HER2 and VEGFR synergistically improves therapeutic efficacy via PI3K-AKT pathway in advanced ovarian cancer.J Exp Clin Cancer Res. 2024 Feb 26;43(1):56. doi: 10.1186/s13046-024-02981-5. J Exp Clin Cancer Res. 2024. PMID: 38403634 Free PMC article.
-
New Insights into Chemoresistance Mediated by Mdm2 Inhibitors: The Benefits of Targeted Therapy over Common Cytostatics.Biomedicines. 2024 Feb 29;12(3):547. doi: 10.3390/biomedicines12030547. Biomedicines. 2024. PMID: 38540160 Free PMC article.
-
Leveraging diverse cell death patterns in osteosarcoma patients and identification of the function of FADS2 in osteosarcoma cells.Sci Rep. 2025 Jul 1;15(1):20831. doi: 10.1038/s41598-025-05480-5. Sci Rep. 2025. PMID: 40593006 Free PMC article.
-
Organoids in ovarian cancer: a platform for disease modeling, precision medicine, and drug assessment.J Cancer Res Clin Oncol. 2024 Mar 20;150(3):146. doi: 10.1007/s00432-024-05654-0. J Cancer Res Clin Oncol. 2024. PMID: 38509422 Free PMC article. Review.
-
FRAX486, a PAK inhibitor, overcomes ABCB1-mediated multidrug resistance in breast cancer cells.Braz J Med Biol Res. 2024 Jul 1;57:e13357. doi: 10.1590/1414-431X2024e13357. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 38958364 Free PMC article.
References
-
- American Cancer Society. Cancer Statistics Center—Ovary 2018 [cited 2020 April 13]. Available from: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Ovary.
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al.. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. Epub 2003/07/16. doi: 10.1200/JCO.2003.02.153 . - DOI - PubMed
-
- Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al.. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92(9):699–708. Epub 2000/05/04. doi: 10.1093/jnci/92.9.699 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

