Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients
- PMID: 34347934
- PMCID: PMC8441872
- DOI: 10.1111/ajt.16766
Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients
Abstract
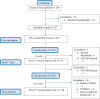
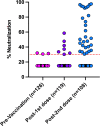
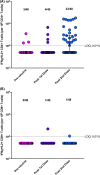
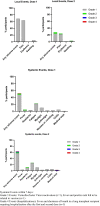
Solid organ transplant recipients are at high risk of severe disease from COVID-19. We assessed the immunogenicity of mRNA-1273 vaccine using a combination of antibody testing, surrogate neutralization assays, and T cell assays. Patients were immunized with two doses of vaccine and immunogenicity assessed after each dose using the above tests. CD4+ and CD8+ T cell responses were assessed in a subset using flow-cytometry. A total of 127 patients were enrolled of which 110 provided serum at all time points. A positive anti-RBD antibody was seen in 5.0% after one dose and 34.5% after two doses. Neutralizing antibody was present in 26.9%. Of note, 28.5% of patients with anti-RBD did not have neutralizing antibody. T cell responses in a sub-cohort of 48 patients showed a positive CD4+ T cell response in 47.9%. Of note, in this sub-cohort, 46.2% of patients with a negative anti-RBD, still had a positive CD4+ T cell response. The vaccine was safe and well-tolerated. In summary, immunogenicity of mRNA-1273 COVID-19 vaccine was modest, but a subset of patients still develop neutralizing antibody and CD4+T- cell responses. Importantly polyfunctional CD4+T cell responses were observed in a significant portion who were antibody negative, further highlighting the importance of vaccination in this patient population. IRB Statement: This study was approved by the University Health Network Research Ethics Board (CAPCR ID 20-6069).
Keywords: clinical research/practice; infection and infectious agents - viral; infectious disease; vaccine.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

References
-
- Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1097 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

