Feedback and Communication in Active Hydrogel Spheres with pH Fronts: Facile Approaches to Grow Soft Hydrogel Structures
- PMID: 34347941
- PMCID: PMC8518392
- DOI: 10.1002/anie.202109735
Feedback and Communication in Active Hydrogel Spheres with pH Fronts: Facile Approaches to Grow Soft Hydrogel Structures
Abstract
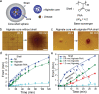
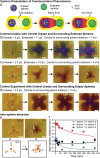
Compartmentalized reaction networks regulating signal processing, communication and pattern formation are central to living systems. Towards achieving life-like materials, we compartmentalized urea-urease and more complex urea-urease/ester-esterase pH-feedback reaction networks into hydrogel spheres and investigate how fuel-driven pH fronts can be sent out from these spheres and regulated by internal reaction networks. Membrane characteristics are installed by covering urease spheres with responsive hydrogel shells. We then encapsulate the two networks (urea-urease and ester-esterase) separately into different hydrogel spheres to devise communication, pattern formation and attraction. Moreover, these pH fronts and patterns can be used for self-growing hydrogels, and for developing complex geometries from non-injectable hydrogels without 3D printing tools. This study opens possibilities for compartmentalized feedback reactions and their use in next generation materials fabrication.
Keywords: chemical reaction networks; hydrogels; life-like systems; pH feedback system; supramolecular chemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Meinhardt H. in Current Topics in Developmental Biology, Vol. 81 (Eds.: Schnell S., Maini P. K., Newman S. A., Newman T. J.), Academic Press, New York, 2008, pp. 1–63.
-
- Bean B. P., Nat. Rev. Neurosci. 2007, 8, 451–465. - PubMed
-
- Epstein I. R., Pojman J. A., An Introduction to Nonlinear Chemical Dynamics: Oscillations, Waves, Patterns, and Chaos, Oxford University Press, New York, 1998.
Publication types
LinkOut - more resources
Full Text Sources

