Accelerated COVID-19 vaccine development: milestones, lessons, and prospects
- PMID: 34348117
- PMCID: PMC8328682
- DOI: 10.1016/j.immuni.2021.07.017
Accelerated COVID-19 vaccine development: milestones, lessons, and prospects
Abstract
The development of effective vaccines to combat infectious diseases is a complex multi-year and multi-stakeholder process. To accelerate the development of vaccines for coronavirus disease 2019 (COVID-19), a novel pathogen emerging in late 2019 and spreading globally by early 2020, the United States government (USG) mounted an operation bridging public and private sector expertise and infrastructure. The success of the endeavor can be seen in the rapid advanced development of multiple vaccine candidates, with several demonstrating efficacy and now being administered around the globe. Here, we review the milestones enabling the USG-led effort, the methods utilized, and ensuing outcomes. We discuss the current status of COVID-19 vaccine development and provide a perspective for how partnership and preparedness can be better utilized in response to future public-health pandemic emergencies.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests B.G. is listed as an inventor of the following patent and patent application: WO/2018/081318 Prefusion Coronavirus Spike Proteins and their use; PCT Patent Application No. PCT/US2021/017709, filed on February 11, 2021, entitled “SARS-CoV-2 VACCINE.” J.M. and B.G. are listed as inventors on the following patent application: U.S. Provisional Patent Application No. 63/140,250, filed on January 21, 2021, entitled “NEWCASTLE DISEASE VIRUS-LIKE PARTICLE DISPLAYING PREFUSION-STABILIZED SARS-COV-2 SPIKE AND ITS USE.”
Figures
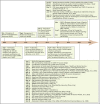
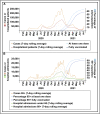
References
-
- Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., Debenham L., Llavall A.C., Dixit A., Zhou D., et al. for PregCOV-19 Living Systematic Review Consortium Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. - PMC - PubMed
-
- Andrejko K., Pry J., Myers J.F., Jewell N.P., Openshaw J., Watt J., Jain S., Lewnard J.A. Early evidence of COVID-19 vaccine effectiveness within the general population of California. medRxiv. 2021 doi: 10.1101/2021.04.08.21255135. - DOI
-
- Aran D. Estimating real-world COVID-19 vaccine effectiveness in Israel using aggregated counts. medRxiv. 2021 doi: 10.1101/2021.02.05.21251139. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

