Slow AMPA receptors in hippocampal principal cells
- PMID: 34348150
- PMCID: PMC8356020
- DOI: 10.1016/j.celrep.2021.109496
Slow AMPA receptors in hippocampal principal cells
Abstract
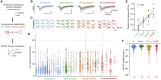
Glutamate receptor ion channels, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, mediate fast excitatory neurotransmission in the CNS. Previous work suggested that AMPA receptors produce a synaptic current with a millisecond duration. However, we find that about two-thirds of principal cells in the hippocampal CA1 region also express AMPA receptors with reduced desensitization that can stay active for half a second after repetitive stimuli. These slow AMPA receptors are expressed at about half of the synapses, with a flat spatial distribution. The increased charge transfer from slow AMPA receptors allows short-term potentiation from a postsynaptic locus and reliable triggering of action potentials. Biophysical and pharmacological observations imply slow AMPA receptors incorporate auxiliary proteins, and their activation lengthens miniature synaptic currents. These data indicate that AMPA receptors are a major source of synaptic diversity. Synapses harboring slow AMPA receptors could have unique roles in hippocampal function.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

