Cost-effectiveness of empagliflozin in patients with type 2 diabetes and established cardiovascular disease in China
- PMID: 34348729
- PMCID: PMC8336098
- DOI: 10.1186/s12962-021-00299-z
Cost-effectiveness of empagliflozin in patients with type 2 diabetes and established cardiovascular disease in China
Abstract
Background: In several cardiovascular outcome trials (CVOTs), empagliflozin (SGLT-2 inhibitor), sitagliptin (DPP-4 inhibitor) and liraglutide (GLP-1 receptor agonist) + standard of care (SoC) were compared to SoC in patients with type 2 diabetes and established cardiovascular disease (CVD). This study assessed the cost-effectiveness (CE) of empagliflozin + SoC in comparison to sitagliptin + SoC and liraglutide + SoC based on the respective CVOT.
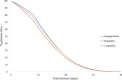
Methods: The IQVIA Core Diabetes Model (CDM) was calibrated to reproduce the CVOT outcomes. EMPA-REG OUTCOME baseline characteristics and CVOT specific treatment effects on risk factors for cardiovascular disease (HbA1c, BMI, blood pressure, lipids) were applied. Three-year observed cardiovascular events of empagliflozin + SoC versus sitagliptin + SoC and liraglutide + SoC were derived from EMPA-REG OUTCOME and an indirect treatment comparison. Relative risk adjustments to calibrate the CDM were obtained after a trial and error process to match as closely the observed and CDM-predicted outcomes. The drug-specific treatment effects were considered up until HbA1c reached 8.5% and treatment switch occurred. After this switch, the United Kingdom Prospective Diabetes Study 82 risk equations predicted events based on co-existing risk factors and treatment intensification to basal bolus insulin were applied. The analysis was conducted from the perspective of the Chinese healthcare system applying 3% discounting. The time horizon was lifelong.
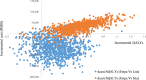
Results: Empagliflozin + SoC provides additional Quality Adjusted Life years (QALY + 0.564) for an incremental cost of 42,497RMB (US$6053) compared to sitagliptin + SoC, resulting in an Incremental Cost Utility Ratio of 75,349RMB (US$10,732), thus below the willingness-to-pay threshold of 212,676RMB, corresponding to three times the Gross Domestic Product in China (2019). Compared to liraglutide + SoC, empagliflozin + SoC use leads to 0.211QALY gained and cost savings of 71,427RMB (US$10,173) and is as such dominant. Scenario and probabilistic sensitivity analyses demonstrated the robustness of the results.
Conclusion: Results suggest that empagliflozin + SoC is cost-effective compared to sitagliptin + SoC and liraglutide + SoC at a willingness-to-pay threshold of 212,676RMB ($30,292)/QALY.
Keywords: Cardiovascular outcomes; Core Diabetes Model; Cost-effectiveness; Empagliflozin; Type 2 diabetes.
© 2021. The Author(s).
Conflict of interest statement
MR, ML are full time employees of IQVIA. IQVIA received consulting fees from Boehringer Ingelheim to conduct this work. AU and XW are full-time employees of Boehringer Ingelheim, the manufacturer of empagliflozin. PM declares no conflict of interest.
Figures


References
-
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

