The exon-junction complex helicase eIF4A3 controls cell fate via coordinated regulation of ribosome biogenesis and translational output
- PMID: 34348895
- PMCID: PMC8336962
- DOI: 10.1126/sciadv.abf7561
The exon-junction complex helicase eIF4A3 controls cell fate via coordinated regulation of ribosome biogenesis and translational output
Abstract
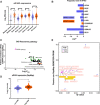
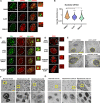
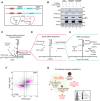
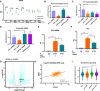
Eukaryotic initiation factor 4A-III (eIF4A3), a core helicase component of the exon junction complex, is essential for splicing, mRNA trafficking, and nonsense-mediated decay processes emerging as targets in cancer therapy. Here, we unravel eIF4A3's tumor-promoting function by demonstrating its role in ribosome biogenesis (RiBi) and p53 (de)regulation. Mechanistically, eIF4A3 resides in nucleoli within the small subunit processome and regulates rRNA processing via R-loop clearance. EIF4A3 depletion induces cell cycle arrest through impaired RiBi checkpoint-mediated p53 induction and reprogrammed translation of cell cycle regulators. Multilevel omics analysis following eIF4A3 depletion pinpoints pathways of cell death regulation and translation of alternative mouse double minute homolog 2 (MDM2) transcript isoforms that control p53. EIF4A3 expression and subnuclear localization among clinical cancer specimens correlate with the RiBi status rendering eIF4A3 an exploitable vulnerability in high-RiBi tumors. We propose a concept of eIF4A3's unexpected role in RiBi, with implications for cancer pathogenesis and treatment.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Peña C., Hurt E., Panse V. G., Eukaryotic ribosome assembly, transport and quality control. Nat. Struct. Mol. Biol. 24, 689–699 (2017). - PubMed
-
- Bursać S., Prodan Y., Pullen N., Bartek J., Volarević S., Dysregulated ribosome biogenesis reveals therapeutic liabilities in cancer. Trends Cancer 7, 57–76 (2021). - PubMed
-
- Khot A., Brajanovski N., Cameron D. P., Hein N., Maclachlan K. H., Sanij E., Lim J., Soong J., Link E., Blombery P., Thompson E. R., Fellowes A., Sheppard K. E., McArthur G. A., Pearson R. B., Hannan R. D., Poortinga G., Harrison S. J., First-in-human RNA polymerase I transcription inhibitor CX-5461 in patients with advanced hematologic cancers: Results of a phase I dose-escalation study. Cancer Discov. 9, 1036–1049 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

