Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: an iSPOT-D report
- PMID: 34349116
- PMCID: PMC8338944
- DOI: 10.1038/s41398-021-01533-1
Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: an iSPOT-D report
Abstract
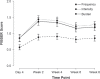
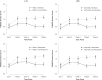
Side effects to antidepressant medications are common and can impact the prognosis of successful treatment outcome in people with major depressive disorder (MDD). However, few studies have investigated the severity of side effects over the course of treatment and their association with treatment outcome. Here we assessed the severity of side effects and the impact of treatment type and anxiety symptoms over the course of treatment, as well as whether side effects were associated with treatment outcome. Participants were N = 1008 adults with a current diagnosis of single-episode or recurrent, nonpsychotic MDD. Participants were randomised to receive escitalopram, sertraline, or venlafaxine-extended release with equal probability and reassessed at 8 weeks regarding Hamilton Rating Scale Depression (HRSD17) and Quick Inventory of Depressive Symptomatology (QIDS-SR16) remission and response. Severity of side effects were assessed using the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale and assessed at day 4 and weeks 2, 4, 6, and 8. Frequency, intensity, and burden of side effects were greatest at week 2, then only frequency and intensity of side effects gradually decreased up to week 6. Treatment type and anxiety symptoms did not impact the severity of side effects. A greater burden-but not frequency or intensity-of side effects was associated with poorer treatment outcome and as early as 4 days post-treatment. Together, this work provides an informative mapping of the progression of side effects throughout the treatment course and their association with treatment outcome. Importantly, the burden of side effects that are present as early as 4 days post-treatment predicts poorer treatment outcome and should be monitored closely. iSPOT-D: Registry name: ClinicalTrials.gov. Registration number: NCT00693849.
© 2021. The Author(s).
Conflict of interest statement
TAB, DMP, and EG report salaries from Total Brain outside the submitted work. EG is the CMO at Total Brain and holds significant equity and stock options in the company. AWFH reports personal fees from Janssen Australia, Lundbeck Australia, Servier Australia, and Takeda Pharmaceutical Company outside the submitted work. AJR has received consulting fees from Akili, Brain Resource Inc., Compass Inc., Curbstone Consultant LLC, Emmes Corp., Holmusk, Inc., Liva-Nova, Johnson & Johnson (Janssen), Sunovion, Takeda USA, and Taj Medical; speaking fees from LivaNova; and royalties from Guilford Press and the University of Texas Southwestern Medical Center, Dallas, TX (for the Inventory of Depressive Symptoms and its derivatives). He is also named co-inventor on two patents: US Patent No. 7,795,033: Methods to predict the outcome of treatment with antidepressant medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S, Wilson AS and US Patent No. 7,906,283: Methods to identify patients at risk of developing adverse events during treatment with antidepressant medication, Inventors: McMahon FJ, Laje G, Manji H, Rush AJ, Paddock S. GT has no conflicts of interest to report.
Figures
References
-
- Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry. 2017;7:58.. doi: 10.1186/s12888-016-1173-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials