Application of alpha1-antitrypsin in a rat model of veno-arterial extracorporeal membrane oxygenation
- PMID: 34349162
- PMCID: PMC8339069
- DOI: 10.1038/s41598-021-95119-y
Application of alpha1-antitrypsin in a rat model of veno-arterial extracorporeal membrane oxygenation
Abstract
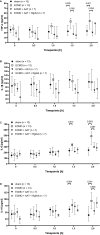
Extracorporeal membrane oxygenation (ECMO) is a life-saving intervention for patients suffering from respiratory or cardiac failure. The ECMO-associated morbidity and mortality depends to a large extent on the underlying disease and is often related to systemic inflammation, consecutive immune paralysis and sepsis. Here we tested the hypothesis that human α1-antitrypsin (SERPINA1) due to its anti-protease and anti-inflammatory functions may attenuate ECMO-induced inflammation. We specifically aimed to test whether intravenous treatment with α1-antitrypsin reduces the release of cytokines in response to 2 h of experimental ECMO. Adult rats were intravenously infused with α1-antitrypsin immediately before starting veno-arterial ECMO. We measured selected pro- and anti-inflammatory cytokines and found, that systemic levels of tumor necrosis factor-α, interleukin-6 and interleukin-10 increase during experimental ECMO. As tachycardia and hypertension developed in response to α1-antitrypsin, a single additional bolus of fentanyl and midazolam was given. Treatment with α1-antitrypsin and higher sedative doses reduced all cytokine levels investigated. We suggest that α1-antitrypsin might have the potential to protect against both ECMO-induced systemic inflammation and immune paralysis. More studies are needed to corroborate our findings, to clarify the mechanisms by which α1-antitrypsin inhibits cytokine release in vivo and to explore the potential application of α1-antitrypsin in clinical ECMO.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Fletcher-Sandersjöö A, Thelin EP, Bartek J, Broman M, Sallisalmi M, Elmi-Terander A, Bellander BM. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: A systematic and narrative review. Front Neurol. 2018;6(9):548. doi: 10.3389/fneur.2018.00548. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

