Manganese systemic distribution is modulated in vivo during tumor progression and affects tumor cell migration and invasion in vitro
- PMID: 34349175
- PMCID: PMC8338990
- DOI: 10.1038/s41598-021-95190-5
Manganese systemic distribution is modulated in vivo during tumor progression and affects tumor cell migration and invasion in vitro
Abstract
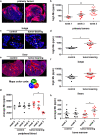
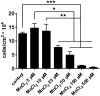
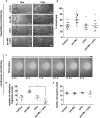
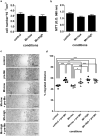
Metastatic disease remains the leading cause of death in cancer and understanding the mechanisms involved in tumor progression continues to be challenging. This work investigates the role of manganese in tumor progression in an in vivo model of tumor growth. Our data revealed that manganese accumulates within primary tumors and secondary organs as manganese-rich niches. Consequences of such phenomenon were investigated, and we verified that short-term changes in manganese alter cell surface molecules syndecan-1 and β1-integrin, enhance collective cell migration and invasive behavior. Long-term increased levels of manganese do not affect cell growth and viability but enhance cell migration. We also observed that manganese is secreted from tumor cells in extracellular vesicles, rather than in soluble form. Finally, we describe exogenous glycosaminoglycans that counteract manganese effects on tumor cell behavior. In conclusion, our analyses describe manganese as a central element in tumor progression by accumulating in Mn-rich niches in vivo, as well as in vitro, affecting migration and extracellular vesicle secretion in vitro. Manganese accumulation in specific regions of the organism may not be a common ground for all cancers, nevertheless, it represents a new aspect of tumor progression that deserves special attention.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

