The application of CRISPR/Cas9 system in cervical carcinogenesis
- PMID: 34349239
- PMCID: PMC9113934
- DOI: 10.1038/s41417-021-00366-w
The application of CRISPR/Cas9 system in cervical carcinogenesis
Erratum in
-
Correction: The application of CRISPR/Cas9 system in cervical carcinogenesis.Cancer Gene Ther. 2022 May;29(5):625-626. doi: 10.1038/s41417-021-00393-7. Cancer Gene Ther. 2022. PMID: 34645976 Free PMC article. No abstract available.
Abstract
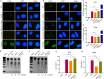
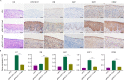
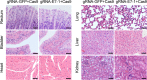
Integration of high-risk HPV genomes into cellular chromatin has been confirmed to promote cervical carcinogenesis, with HPV16 being the most prevalent high-risk type. Herein, we evaluated the therapeutic effect of the CRISPR/Cas9 system in cervical carcinogenesis, especially for cervical precancerous lesions. In cervical cancer/pre-cancer cell lines, we transfected the HPV16 E7 targeted CRISPR/Cas9, TALEN, ZFN plasmids, respectively. Compared to previous established ZFN and TALEN systems, CRISPR/Cas9 has shown comparable efficiency and specificity in inhibiting cell growth and colony formation and inducing apoptosis in cervical cancer/pre-cancer cell lines, which seemed to be more pronounced in the S12 cell line derived from the low-grade cervical lesion. Furthermore, in xenograft formation assays, CRISPR/Cas9 inhibited tumor formation of the S12 cell line in vivo and affected the corresponding protein expression. In the K14-HPV16 transgenic mice model of HPV-driven spontaneous cervical carcinogenesis, cervical application of CRISPR/Cas9 treatment caused mutations of the E7 gene and restored the expression of RB, E2F1, and CDK2, thereby reversing the cervical carcinogenesis phenotype. In this study, we have demonstrated that CRISPR/Cas9 targeting HPV16 E7 could effectively revert the HPV-related cervical carcinogenesis in vitro, as well as in K14-HPV16 transgenic mice, which has shown great potential in clinical treatment for cervical precancerous lesions.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang SS, Zuna RE, Wentzensen N, Dunn ST, Sherman ME, Gold MA, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomark Prev. 2009;18:113–20. doi: 10.1158/1055-9965.EPI-08-0591. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

