Genetic insights into biological mechanisms governing human ovarian ageing
- PMID: 34349265
- PMCID: PMC7611832
- DOI: 10.1038/s41586-021-03779-7
Genetic insights into biological mechanisms governing human ovarian ageing
Abstract
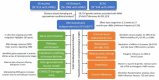
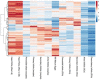
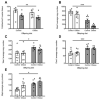
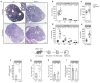
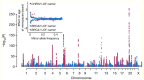
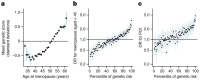
Reproductive longevity is essential for fertility and influences healthy ageing in women1,2, but insights into its underlying biological mechanisms and treatments to preserve it are limited. Here we identify 290 genetic determinants of ovarian ageing, assessed using normal variation in age at natural menopause (ANM) in about 200,000 women of European ancestry. These common alleles were associated with clinical extremes of ANM; women in the top 1% of genetic susceptibility have an equivalent risk of premature ovarian insufficiency to those carrying monogenic FMR1 premutations3. The identified loci implicate a broad range of DNA damage response (DDR) processes and include loss-of-function variants in key DDR-associated genes. Integration with experimental models demonstrates that these DDR processes act across the life-course to shape the ovarian reserve and its rate of depletion. Furthermore, we demonstrate that experimental manipulation of DDR pathways highlighted by human genetics increases fertility and extends reproductive life in mice. Causal inference analyses using the identified genetic variants indicate that extending reproductive life in women improves bone health and reduces risk of type 2 diabetes, but increases the risk of hormone-sensitive cancers. These findings provide insight into the mechanisms that govern ovarian ageing, when they act, and how they might be targeted by therapeutic approaches to extend fertility and prevent disease.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Full individual study and author disclosures can be found in the Supplementary Information.
Figures








Comment in
-
Genomic analysis identifies variants that can predict the timing of menopause.Nature. 2021 Aug;596(7872):345-346. doi: 10.1038/d41586-021-01710-8. Nature. 2021. PMID: 34349269 No abstract available.
-
Genetics of female reproductive longevity.Nat Rev Endocrinol. 2021 Oct;17(10):575. doi: 10.1038/s41574-021-00558-2. Nat Rev Endocrinol. 2021. PMID: 34408296 No abstract available.
-
Dissecting the genetics of ovarian ageing.Nat Rev Genet. 2021 Oct;22(10):623. doi: 10.1038/s41576-021-00415-y. Nat Rev Genet. 2021. PMID: 34446918 No abstract available.
References
-
- Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ. Testing ovarian reserve to predict age at menopause. Maturitas. 2009;63:280–91. - PubMed
MeSH terms
Substances
Grants and funding
- P30 ES010126/ES/NIEHS NIH HHS/United States
- 212946/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- 16565/CRUK_/Cancer Research UK/United Kingdom
- P50 CA058223/CA/NCI NIH HHS/United States
- T32 CA057726/CA/NCI NIH HHS/United States
- MC_UU_00011/6/MRC_/Medical Research Council/United Kingdom
- R01 GM098605/GM/NIGMS NIH HHS/United States
- 204623/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 29019/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00007/10/MRC_/Medical Research Council/United Kingdom
- MC_U137686851/MRC_/Medical Research Council/United Kingdom
- MC_PC_17189/MRC_/Medical Research Council/United Kingdom
- 16896/CRUK_/Cancer Research UK/United Kingdom
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- 29186/CRUK_/Cancer Research UK/United Kingdom
- U01 HL130114/HL/NHLBI NIH HHS/United States
- MC_UU_12012/4/MRC_/Medical Research Council/United Kingdom
- MC_UU_00011/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12026/2/MRC_/Medical Research Council/United Kingdom
- G1001357/MRC_/Medical Research Council/United Kingdom
- R01 HL105756/HL/NHLBI NIH HHS/United States
- MC_UU_00002/7/MRC_/Medical Research Council/United Kingdom
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- MC_PC_14135/MRC_/Medical Research Council/United Kingdom
- 16563/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00017/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- U01 CA182913/CA/NCI NIH HHS/United States
- MC_UU_00006/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00006/2/MRC_/Medical Research Council/United Kingdom
- 202802/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 10118/CRUK_/Cancer Research UK/United Kingdom
- 16561/CRUK_/Cancer Research UK/United Kingdom
- MC_PC_13049/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/4/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
- Andalusian Health Repository - access to free full text
- Archivio Istituzionale della Ricerca Unimi - Access Free Full Text
- Europe PubMed Central
- HAL archives ouvertes - Access Free Full Text
- Nature Publishing Group
- PubMed Central
- White Rose Research Online
- eScholarship, University of California - Access Free Full Text
Other Literature Sources
Medical
Molecular Biology Databases

