Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer's Disease
- PMID: 34349624
- PMCID: PMC8326917
- DOI: 10.3389/fncel.2021.695479
Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer's Disease
Abstract
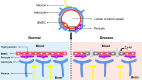
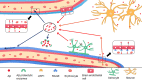
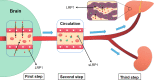
Amyloid-β (Aβ) is the predominant pathologic protein in Alzheimer's disease (AD). The production and deposition of Aβ are important factors affecting AD progression and prognosis. The deposition of neurotoxic Aβ contributes to damage of the blood-brain barrier. However, the BBB is also crucial in maintaining the normal metabolism of Aβ, and dysfunction of the BBB aggravates Aβ deposition. This review characterizes Aβ deposition and BBB damage in AD, summarizes their interactions, and details their respective mechanisms.
Keywords: Alzheimer’s disease; P-glycoprotein; amyloid-β; blood–brain barrier; low-density lipoprotein receptor-related protein 1; receptor for advanced glycation end products.
Copyright © 2021 Wang, Chen, Han, Yin, Ge and Lei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

