Arm Ergometry to Improve Mobility in Progressive Multiple Sclerosis (AMBOS)-Results of a Pilot Randomized Controlled Trial
- PMID: 34349716
- PMCID: PMC8326796
- DOI: 10.3389/fneur.2021.644533
Arm Ergometry to Improve Mobility in Progressive Multiple Sclerosis (AMBOS)-Results of a Pilot Randomized Controlled Trial
Abstract
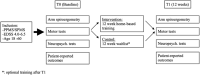
Background: Walking disability is one of the most frequent and burdening symptoms of progressive multiple sclerosis (MS). Most of the exercise intervention studies that showed an improvement in mobility performance were conducted in low to moderately disabled relapsing-remitting MS patients with interventions using the legs. However, MS patients with substantial walking disability hardly can perform these tasks. Earlier work has indicated that aerobic arm training might also improve walking performance and could therefore be a therapeutic option in already moderately disabled progressive MS patients. Methods: Patients with progressive MS and EDSS 4-6.5 were randomized using a computer-generated algorithm list to either a waitlist control group (CG) or an intervention group (IG). The IG performed a 12-week home-based, individualized arm ergometry exercise training program. Maximum walking distance as measured by the 6-min walking test (6MWT) was the primary endpoint. Secondary endpoints included aerobic fitness, other mobility tests, cognitive functioning, as well as fatigue and depression. Results: Of n = 86 screened patients, 53 with moderate disability (mean EDSS 5.5, SD 0.9) were included and data of 39 patients were analyzed. Patients in the IG showed strong adherence to the program with a mean of 67 (SD 26.4) training sessions. Maximum work load (P max) increased in the training group while other fitness indicators did not. Walking distance in the 6MWT improved in both training and waitlist group but not significantly more in trained patients. Similarly, other mobility measures showed no differential group effect. Cognitive functioning remained unchanged. No serious events attributable to the intervention occurred. Conclusion: Although maximum work load improved, 3 months of high-frequency arm ergometry training of low to moderate intensity could not show improved walking ability or cognitive functioning in progressive MS compared to a waitlist CG. The study was registered at www.clinicaltrials.gov (NCT03147105) and funded by the local MS self-help organization.
Keywords: aerobic exercise; arm ergometry; cognition; multiple sclerosis; progressive multiple sclerosis.
Copyright © 2021 Heinrich, Rosenthal, Patra, Schulz, Welsch, Vettorazzi, Rosenkranz, Stellmann, Ramien, Pöttgen, Gold and Heesen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Erratum.Mult Scler. 2016 Oct;22(12):NP9-NP11. doi: 10.1177/1352458515585718. Epub 2015 Jun 3. Mult Scler. 2016. PMID: 26041800
-
Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial.Mult Scler. 2014 Mar;20(3):382-90. doi: 10.1177/1352458513507358. Epub 2013 Oct 24. Mult Scler. 2014. PMID: 24158978 Clinical Trial.
-
The effectiveness of Robot-Assisted Gait Training versus conventional therapy on mobility in severely disabled progressIve MultiplE sclerosis patients (RAGTIME): study protocol for a randomized controlled trial.Trials. 2017 Feb 27;18(1):88. doi: 10.1186/s13063-017-1838-2. Trials. 2017. PMID: 28241776 Free PMC article. Clinical Trial.
-
Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations.BMC Neurol. 2017 Sep 16;17(1):185. doi: 10.1186/s12883-017-0960-9. BMC Neurol. 2017. PMID: 28915856 Free PMC article. Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Safety of exercise training in multiple sclerosis: An updated systematic review and meta-analysis.Mult Scler. 2023 Nov;29(13):1604-1631. doi: 10.1177/13524585231204459. Epub 2023 Oct 26. Mult Scler. 2023. PMID: 37880997 Free PMC article.
-
Gait Variability as a Potential Motor Marker of Cerebellar Disease-Relationship between Variability of Stride, Arm Swing and Trunk Movements, and Walking Speed.Sensors (Basel). 2024 May 28;24(11):3476. doi: 10.3390/s24113476. Sensors (Basel). 2024. PMID: 38894268 Free PMC article.
-
Translation and validation of the multiple sclerosis walking scale 12 for the German population - the MSWS-12/D.Health Qual Life Outcomes. 2023 Oct 9;21(1):110. doi: 10.1186/s12955-023-02190-2. Health Qual Life Outcomes. 2023. PMID: 37814258 Free PMC article.
References
-
- Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA, et al. . Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. (2013) 94:1800–28.e3. 10.1016/j.apmr.2013.04.020 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

