Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients
- PMID: 34349747
- PMCID: PMC8326578
- DOI: 10.3389/fmicb.2021.705020
Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients
Abstract
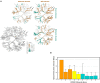
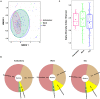
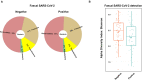
The risk factors for coronavirus disease 2019 (COVID-19) severity are still poorly understood. Considering the pivotal role of the gut microbiota on host immune and inflammatory functions, we investigated the association between changes in the gut microbiota composition and the clinical severity of COVID-19. We conducted a multicenter cross-sectional study prospectively enrolling 115 COVID-19 patients categorized according to: (1) the WHO Clinical Progression Scale-mild, 19 (16.5%); moderate, 37 (32.2%); or severe, 59 (51.3%), and (2) the location of recovery from COVID-19-ambulatory, 14 (household isolation, 12.2%); hospitalized in ward, 40 (34.8%); or hospitalized in the intensive care unit, 61 (53.0%). Gut microbiota analysis was performed through 16S rRNA gene sequencing, and the data obtained were further related to the clinical parameters of COVID-19 patients. The risk factors for COVID-19 severity were identified by univariate and multivariable logistic regression models. In comparison to mild COVID-19 patients, the gut microbiota of moderate and severe patients have: (a) lower Firmicutes/Bacteroidetes ratio; (b) higher abundance of Proteobacteria; and (c) lower abundance of beneficial butyrate-producing bacteria such as the genera Roseburia and Lachnospira. Multivariable regression analysis showed that the Shannon diversity index [odds ratio (OR) = 2.85, 95% CI = 1.09-7.41, p = 0.032) and C-reactive protein (OR = 3.45, 95% CI = 1.33-8.91, p = 0.011) are risk factors for severe COVID-19 (a score of 6 or higher in the WHO Clinical Progression Scale). In conclusion, our results demonstrated that hospitalized patients with moderate and severe COVID-19 have microbial signatures of gut dysbiosis; for the first time, the gut microbiota diversity is pointed out as a prognostic biomarker of COVID-19 severity.
Keywords: COVID-19; Shannon—Weiner diversity index; WHO Clinical Progression Scale; dysbiosis; gut microbiota.
Copyright © 2021 Moreira-Rosário, Marques, Pinheiro, Araújo, Ribeiro, Rocha, Mota, Pestana, Ribeiro, Pereira, de Sousa, Pereira-Leal, de Sousa, Morais, Teixeira, Rocha, Silvestre, Príncipe, Gatta, Amado, Santos, Maltez, Boquinhas, de Sousa, Germano, Sarmento, Granja, Póvoa, Faria and Calhau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

