AeMOPE-1, a Novel Salivary Peptide From Aedes aegypti, Selectively Modulates Activation of Murine Macrophages and Ameliorates Experimental Colitis
- PMID: 34349757
- PMCID: PMC8327214
- DOI: 10.3389/fimmu.2021.681671
AeMOPE-1, a Novel Salivary Peptide From Aedes aegypti, Selectively Modulates Activation of Murine Macrophages and Ameliorates Experimental Colitis
Abstract
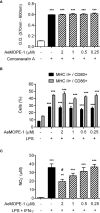
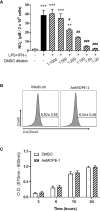
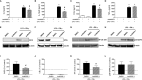
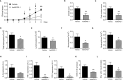
The sialotranscriptomes of Aedes aegypti revealed a transcript overexpressed in female salivary glands that codes a mature 7.8 kDa peptide. The peptide, specific to the Aedes genus, has a unique sequence, presents a putative secretory nature and its function is unknown. Here, we confirmed that the peptide is highly expressed in the salivary glands of female mosquitoes when compared to the salivary glands of males, and its secretion in mosquito saliva is able to sensitize the vertebrate host by inducing the production of specific antibodies. The synthetic version of the peptide downmodulated nitric oxide production by activated peritoneal murine macrophages. The fractionation of a Ae. aegypti salivary preparation revealed that the fractions containing the naturally secreted peptide reproduced the nitric oxide downmodulation. The synthetic peptide also selectively interfered with cytokine production by murine macrophages, inhibiting the production of IL-6, IL-12p40 and CCL2 without affecting TNF-α or IL-10 production. Likewise, intracellular proteins associated with macrophage activation were also distinctively modulated: while iNOS and NF-κB p65 expression were diminished, IκBα and p38 MAPK expression did not change in the presence of the peptide. The anti-inflammatory properties of the synthetic peptide were tested in vivo on a dextran sulfate sodium-induced colitis model. The therapeutic administration of the Ae. aegypti peptide reduced the leukocytosis, macrophage activity and nitric oxide levels in the gut, as well as the expression of cytokines associated with the disease, resulting in amelioration of its clinical signs. Given its biological properties in vitro and in vivo, the molecule was termed Aedes-specific MOdulatory PEptide (AeMOPE-1). Thus, AeMOPE-1 is a novel mosquito-derived immunobiologic with potential to treat immune-mediated disorders.
Keywords: AeMOPE-1; Aedes aegypti; experimental colitis; immunomodulation; macrophages.
Copyright © 2021 Lara, Esteves, Sales-Campos, Assis, Henrique, Barros, Neto, Silva, Martins, Cardoso, Ribeiro and Sá-Nunes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Aedes aegypti saliva impairs M1-associated proinflammatory phenotype without promoting or affecting M2 polarization of murine macrophages.Parasit Vectors. 2019 May 16;12(1):239. doi: 10.1186/s13071-019-3487-7. Parasit Vectors. 2019. PMID: 31097013 Free PMC article.
-
Analysis in a murine model points to IgG responses against the 34k2 salivary proteins from Aedes albopictus and Aedes aegypti as novel promising candidate markers of host exposure to Aedes mosquitoes.PLoS Negl Trop Dis. 2019 Oct 16;13(10):e0007806. doi: 10.1371/journal.pntd.0007806. eCollection 2019 Oct. PLoS Negl Trop Dis. 2019. PMID: 31618201 Free PMC article.
-
Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus.Med Vet Entomol. 2004 Jun;18(2):191-9. doi: 10.1111/j.1365-2915.2004.00498.x. Med Vet Entomol. 2004. PMID: 15189245
-
Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function.Parasite Immunol. 2004 Jun-Jul;26(6-7):295-306. doi: 10.1111/j.0141-9838.2004.00712.x. Parasite Immunol. 2004. PMID: 15541033
-
Advances in mosquito allergy.Curr Opin Allergy Clin Immunol. 2007 Aug;7(4):350-4. doi: 10.1097/ACI.0b013e328259c313. Curr Opin Allergy Clin Immunol. 2007. PMID: 17620829 Review.
Cited by
-
Peptidomics Analysis Reveals the Buccal Gland of Jawless Vertebrate Lamprey as a Source of Multiple Bioactive Peptides.Mar Drugs. 2023 Jun 29;21(7):389. doi: 10.3390/md21070389. Mar Drugs. 2023. PMID: 37504920 Free PMC article.
-
Aedes aegypti salivary gland extract alleviates acute itching by blocking TRPA1 channels.Front Physiol. 2023 Jun 27;14:1055706. doi: 10.3389/fphys.2023.1055706. eCollection 2023. Front Physiol. 2023. PMID: 37441000 Free PMC article.
-
Amblyostatin-1, the first salivary cystatin with host immunomodulatory and anti-inflammatory properties from the Neotropical tick Amblyomma sculptum, vector of Brazilian spotted fever.Front Immunol. 2025 Jul 17;16:1585703. doi: 10.3389/fimmu.2025.1585703. eCollection 2025. Front Immunol. 2025. PMID: 40746554 Free PMC article.
References
-
- Calvo E, Tokumasu F, Marinotti O, Villeval JL, Ribeiro JM, Francischetti IM. Aegyptin, A Novel Mosquito Salivary Gland Protein, Specifically Binds to Collagen and Prevents Its Interaction With Platelet Glycoprotein VI, Integrin alpha2beta1, and Von Willebrand Factor. J Biol Chem (2007) 282(37):26928–38. 10.1074/jbc.M705669200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

