Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors
- PMID: 34349760
- PMCID: PMC8328433
- DOI: 10.3389/fimmu.2021.694853
Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors
Abstract
Low-molecular weight chemical compounds have a longstanding history as drugs. Target specificity and binding efficiency represent major obstacles for small molecules to become clinically relevant. Protein kinases are attractive cellular targets; however, they are challenging because they present one of the largest protein families and share structural similarities. Bruton tyrosine kinase (BTK), a cytoplasmic protein tyrosine kinase, has received much attention as a promising target for the treatment of B-cell malignancies and more recently autoimmune and inflammatory diseases. Here we describe the structural properties and binding modes of small-molecule BTK inhibitors, including irreversible and reversible inhibitors. Covalently binding compounds, such as ibrutinib, acalabrutinib and zanubrutinib, are discussed along with non-covalent inhibitors fenebrutinib and RN486. The focus of this review is on structure-function relationships.
Keywords: BTK inhibitors; acalabrutinib; covalent and non-covalent binding; fenebrutinib; ibrutinib; protein-inhibitor interactions; structure-function relationship; zanubrutinib.
Copyright © 2021 Zain and Vihinen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

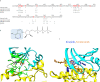

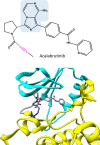
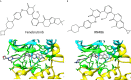
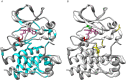
References
-
- Bruton OC. Agammaglobulinemia. Pediatrics (1952) 9(6):722–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

