Case Report: Local Cytokine Release Syndrome in an Acute Lymphoblastic Leukemia Patient After Treatment With Chimeric Antigen Receptor T-Cell Therapy: A Possible Model, Literature Review and Perspective
- PMID: 34349766
- PMCID: PMC8326907
- DOI: 10.3389/fimmu.2021.707191
Case Report: Local Cytokine Release Syndrome in an Acute Lymphoblastic Leukemia Patient After Treatment With Chimeric Antigen Receptor T-Cell Therapy: A Possible Model, Literature Review and Perspective
Abstract
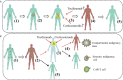
Chimeric antigen receptor T (CAR-T) cell therapy has achieved remarkable clinical efficacy in treatment of many malignancies especially for B-cell hematologic malignancies. However, the application of CAR-T cells is hampered by potentially adverse events, of which cytokine release syndrome (CRS) is one of the severest and the most studied. Local cytokine-release syndrome (L-CRS) at particular parts of the body has been reported once in a while in B-cell lymphoma or other compartmental tumors. The underlying mechanism of L-CRS is not well understood and the existing reports attempting to illustrate it only involve compartmental tumors, some of which even indicated L-CRS only happens in compartmental tumors. Acute lymphoblastic leukemia (ALL) is systemic and our center treated a B-cell ALL patient who exhibited life threatening dyspnea, L-CRS was under suspicion and the patient was successfully rescued with treatment algorithm of CRS. The case is the firstly reported L-CRS related to systemic malignancies and we tentatively propose a model to illustrate the occurrence and development of L-CRS of systemic malignancies inspired by the case and literature, with emphasis on the new recognition of L-CRS.
Keywords: acute lymphoblastic leukemia; chimeric antigen receptor T therapy; local cytokine-release syndrome; possible model; systemic cytokine-release syndrome.
Copyright © 2021 Luan, Zhou, Wang, Ma, Long, Cheng, Chen, Huang, Zhang, Xia and Ge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia.J Cell Mol Med. 2021 Dec;25(24):11063-11074. doi: 10.1111/jcmm.17029. Epub 2021 Nov 3. J Cell Mol Med. 2021. PMID: 34734474 Free PMC article.
-
Individual Patient Data Meta-Analysis from 16 Trials for Safety Factors in Cytokine Release Syndrome After CAR-T Therapy in Patients with Non-Hodgkin Lymphoma (NHL) and Acute Lymphoblastic Leukemia.Adv Ther. 2019 Oct;36(10):2881-2894. doi: 10.1007/s12325-019-01056-8. Epub 2019 Aug 19. Adv Ther. 2019. PMID: 31428935
-
Using JAK inhibitor to treat cytokine release syndrome developed after chimeric antigen receptor T cell therapy for patients with refractory acute lymphoblastic leukemia: A case report.Medicine (Baltimore). 2021 May 14;100(19):e25786. doi: 10.1097/MD.0000000000025786. Medicine (Baltimore). 2021. PMID: 34106613 Free PMC article.
-
Management of cytokine release syndrome related to CAR-T cell therapy.Front Med. 2019 Oct;13(5):610-617. doi: 10.1007/s11684-019-0714-8. Epub 2019 Sep 28. Front Med. 2019. PMID: 31571160
-
Cytokine release syndrome after CAR T-cell therapy for B-cell acute lymphoblastic leukemia in children and young adolescents: storms make trees take deeper roots.Expert Opin Pharmacother. 2024 Aug;25(11):1497-1506. doi: 10.1080/14656566.2024.2387673. Epub 2024 Aug 6. Expert Opin Pharmacother. 2024. PMID: 39087712 Review.
Cited by
-
CT Demonstration of Local Cytokine-Release Syndrome Involving the Head and Neck Following Chimeric Antigen Receptor T Cell Infusion Therapy.Korean J Radiol. 2024 Apr;25(4):399-402. doi: 10.3348/kjr.2023.1100. Korean J Radiol. 2024. PMID: 38528698 Free PMC article. No abstract available.
-
Salivary gland swelling as a characteristic manifestation of local cytokine release syndrome after anti-CD19 chimeric antigen receptor T cell therapy: A case series.J Clin Exp Hematop. 2024;64(3):261-267. doi: 10.3960/jslrt.24035. J Clin Exp Hematop. 2024. PMID: 39343612 Free PMC article.
-
Severe cases of local cytokine release syndrome (CRS); craniocervical edema soon after chimeric antigen T-cell (CAR-T) therapy.Oxf Med Case Reports. 2025 Jan 18;2025(1):omae164. doi: 10.1093/omcr/omae164. eCollection 2025 Jan. Oxf Med Case Reports. 2025. PMID: 39839700 Free PMC article.
-
Efficacy and safety of CD19 CAR-T cell therapy for patients with B cell acute lymphoblastic leukemia involving extramedullary relapse.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Apr 25;51(2):151-159. doi: 10.3724/zdxbyxb-2022-0036. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36161297 Free PMC article. English.
-
A Case Report of Sustained Cytokine Release Syndrome Due to Glofitamab and Literature Review.Clin Pharmacol. 2025 Apr 29;17:79-83. doi: 10.2147/CPAA.S515122. eCollection 2025. Clin Pharmacol. 2025. PMID: 40322275 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

