Isoprenoid Metabolism and Engineering in Glandular Trichomes of Lamiaceae
- PMID: 34349773
- PMCID: PMC8326662
- DOI: 10.3389/fpls.2021.699157
Isoprenoid Metabolism and Engineering in Glandular Trichomes of Lamiaceae
Abstract
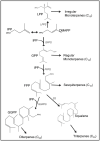
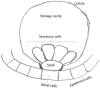
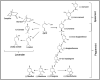
The isoprenoids play important ecological and physiological roles in plants. They also have a tremendous impact on human lives as food additives, medicines, and industrial raw materials, among others. Though some isoprenoids are highly abundant in nature, plants produce many at extremely low levels. Glandular trichomes (GT), which cover the aerial parts of more than 25% of vascular plants, have been considered as natural biofactories for the mass production of rare industrially important isoprenoids. In several plant genera (e.g., Lavandula and Mentha), GTs produce and store large quantities of the low molecular weight isoprenoids, in particular mono- and sesquiterpenes, as essential oil constituents. Within each trichome, a group of secretory cells is specialized to strongly and specifically express isoprenoid biosynthetic genes, and to synthesize and deposit copious amounts of terpenoids into the trichome's storage reservoir. Despite the abundance of certain metabolites in essential oils and defensive resins, plants, particularly those lacking glandular trichomes, accumulate small quantities of many of the biologically active and industrially important isoprenoids. Therefore, there is a pressing need for technologies to enable the mass production of such metabolites, and to help meet the ever-increasing demand for plant-based bioproducts, including medicines and renewable materials. Considerable contemporary research has focused on engineering isoprenoid metabolism in GTs, with the goal of utilizing them as natural biofactories for the production of valuable phytochemicals. In this review, we summarize recent advances related to the engineering of isoprenoid biosynthetic pathways in glandular trichomes.
Keywords: glandular trichomes; isoprenoids; lavender; metabolic engineering; mint.
Copyright © 2021 Mahmoud, Maddock and Adal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Birchfield A. S., McIntosh C. A. (2020). Metabolic engineering and synthetic biology of plant natural products—A minireview. Curr. Plant Biol. 24:100163. 10.1016/j.cpb.2020.100163 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

