A propensity score-weighted comparison between adalimumab originator and its biosimilars, ABP501 and SB5, in inflammatory bowel disease: a multicenter Italian study
- PMID: 34349836
- PMCID: PMC8295962
- DOI: 10.1177/17562848211031420
A propensity score-weighted comparison between adalimumab originator and its biosimilars, ABP501 and SB5, in inflammatory bowel disease: a multicenter Italian study
Abstract
Background: Adalimumab is an effective and safe biological drug for the treatment of inflammatory bowel disease (IBD). Nowadays, several biosimilar agents are available, but data regarding their efficacy and safety in patients with IBD are still lacking. We aimed to compare the effectiveness and tolerability between adalimumab originator, ABP501 and SB5 biosimilars in patients with IBD in the short term (after induction and after 6 months of treatment) through a propensity score-weighted multicenter cohort study.
Methods: We included 156 patients with IBD, 69 patients with ulcerative colitis and 87 patients with Crohn's disease (CD) receiving ABP501 or SB5 biosimilars from January 2019 to April 2020 for moderate-to-severe disease. For comparison, a group of age- and sex-matched patients treated with adalimumab originator was used. We collected clinical and biochemical data after induction and at 6 months of treatment. Endoscopic data were recorded only at baseline.
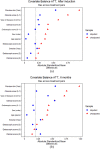
Results: Overall, clinical benefit was achieved by 86.4% and 85.3% after induction and at 6 months, respectively, without a statistically significant difference between the three treatment groups (p = 0.68 and p = 0.46). However, after induction, we found significant differences between the two types of the disease (ulcerative colitis or CD, p = 0.004), with a greater clinical benefit achieved by patients with CD. Also, the therapeutic optimization rate between the three drugs was not statistically significant different (p = 0.30). All treatments showed a good safety profile, with only 10 patients who needed to stop therapy because of adverse events.
Conclusion: Adalimumab biosimilars seem to be as effective and safe as the originator in patients with IBD. Surely, they represent a great opportunity to reduce the costs of biological therapies, however larger and longer real-life studies are necessary.
Keywords: ABP501; Crohn’s disease; SB5; adalimumab; anti-TNF; biosimilar; inflammatory bowel disease; ulcerative colitis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: EVS has received lecture or consultancy fees from Takeda, Merck & Co, Bristol-Myers Squibb, Abbvie, Amgen, Novartis, Fresenius Kabi, Sandoz, Sofar and Janssen. FZ had received lecture fees from Takeda, Janssen and Norgine. The remaining authors declare no competing interests.
Figures

References
-
- Maaser C, Sturm A, Vavricka SR, et al.. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohn’s Colitis 2019; 13: 144–164. - PubMed
-
- Barberio B, Zingone F, Savarino EV. Inflammatory bowel disease and sleep disturbance: as usual, quality matters. Dig Dis Sci 2020; 66: 3–4. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

