Highly efficient on-DNA amide couplings promoted by micelle forming surfactants for the synthesis of DNA encoded libraries
- PMID: 34349922
- PMCID: PMC8278914
- DOI: 10.1039/d1sc03007h
Highly efficient on-DNA amide couplings promoted by micelle forming surfactants for the synthesis of DNA encoded libraries
Abstract
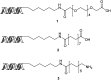
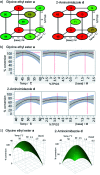
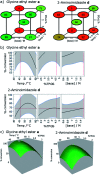
DNA encoded libraries (DELs) represent powerful new technology for finding small molecule ligands for proteins and are increasingly being applied to hit finding in medicinal chemistry. Crucial to the synthesis of high quality DELs is the identification of chemical reactions for their assembly that proceed with very high conversion across a range of different substrates, under conditions compatible with DNA-tagged substrates. Many current chemistries used in DEL synthesis do not meet this requirement, resulting in libraries of low fidelity. Amide couplings are the most commonly used reaction in synthesis of screening libraries and also in DELs. The ability to carry out highly efficient, widely applicable amide couplings in DEL synthesis would therefore be highly desirable. We report a method for amide coupling using micelle forming surfactants, promoted by a modified linker, that is broadly applicable across a wide range of substrates. Most significantly, this works exceptionally well for coupling of DNA-conjugated carboxylic acids (N-to-C) with amines in solution, a procedure that is currently very inefficient. The optimisation of separate procedures for coupling of DNA-conjugated acids and amines by reagent screening and statistically driven optimisation is described. The generality of the method is illustrated by the application to a wide range of examples with unprecedented levels of conversion. The utility of the (N-to-C) coupling of DNA-conjugated acids in DEL synthesis is illustrated by the three cycle synthesis of a fully DNA-encoded compound by two cycles of coupling of an aminoester, with intermediate ester hydrolysis, followed by capping with an amine. This methodology will be of great utility in the synthesis of high fidelity DELs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Witty D. R. and Cox B., Progress in Medicinal Chemistry, Elsevier Science, 2020
-
- Clark M. A. Acharya R. A. Arico-Muendel C. C. Belyanskaya S. L. Benjamin D. R. Carlson N. R. Centrella P. A. Chiu C. H. Creaser S. P. Cuozzo J. W. Davie C. P. Ding Y. Franklin G. J. Franzen K. D. Gefter M. L. Hale S. P. Hansen N. J. V. Israel D. I. Jiang J. Kavarana M. J. Kelley M. S. Kollmann C. S. Li F. Lind K. Mataruse S. Medeiros P. F. Messer J. A. Myers P. O'Keefe H. Oliff M. C. Rise C. E. Satz A. L. Skinner S. R. Svendsen J. L. Tang L. van Vloten K. Wagner R. W. Yao G. Zhao B. Morgan B. A. Nat. Chem. Biol. 2009;5:647–654. doi: 10.1038/nchembio.211. - DOI - PubMed
-
- Soutter H. H. Centrella P. Clark M. A. Cuozzo J. W. Dumelin C. E. Guie M.-A. Habeshian S. Keefe A. D. Kennedy K. M. Sigel E. A. Troast D. M. Zhang Y. Ferguson A. D. Davies G. Stead E. R. Breed J. Madhavapeddi P. Read J. A. Proc. Natl. Acad. Sci. 2016;113:E7880–E7889. doi: 10.1073/pnas.1610978113. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

