The Use of Whole Genome and Exome Sequencing for Newborn Screening: Challenges and Opportunities for Population Health
- PMID: 34350142
- PMCID: PMC8326411
- DOI: 10.3389/fped.2021.663752
The Use of Whole Genome and Exome Sequencing for Newborn Screening: Challenges and Opportunities for Population Health
Abstract
Newborn screening (NBS) is a population-based program with a goal of reducing the burden of disease for conditions with significant clinical impact on neonates. Screening tests were originally developed and implemented one at a time, but newer methods have allowed the use of multiplex technologies to expand additions more rapidly to standard panels. Recent improvements in next-generation sequencing are also evolving rapidly from first focusing on individual genes, then panels, and finally all genes as encompassed by whole exome and genome sequencing. The intersection of these two technologies brings the revolutionary possibility of identifying all genetic disorders in newborns, allowing implementation of therapies at the optimum time regardless of symptoms. This article reviews the history of newborn screening and early studies examining the use of whole genome and exome sequencing as a screening tool. Lessons learned from these studies are discussed, along with technical, ethical, and societal challenges to broad implementation.
Keywords: newborn screening; next-generation sequencing; recommended uniform screening panel; whole exome sequencing; whole genome sequencing.
Copyright © 2021 Woerner, Gallagher, Vockley and Adhikari.
Conflict of interest statement
AA is an employee of Illumina, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
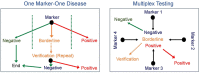
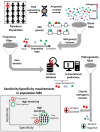
References
-
- Jervis GA. Phenylpyruvic oligophrenia: introductory study of fifty cases of mental deficiency associated with excretion of phenylpyruvic acid. Arch Neurol Psychiatry. (1937) 38:944–63. 10.1001/archneurpsyc.1937.02260230042003 - DOI
-
- Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. (1963) 32:338–43. - PubMed
-
- Blood Testing of Newborn Diseases . Code of Massachusetts Regulation.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

