Patient characteristics and acute cardiovascular event rates among patients with very high-risk and non-very high-risk atherosclerotic cardiovascular disease
- PMID: 34351003
- PMCID: PMC8495090
- DOI: 10.1002/clc.23706
Patient characteristics and acute cardiovascular event rates among patients with very high-risk and non-very high-risk atherosclerotic cardiovascular disease
Abstract
Background: The risk for subsequent major cardiovascular (CV) events among patients with very high-risk (VHR) atherosclerotic CV disease (ASCVD) remains to be fully elucidated.
Hypothesis: We assessed the characteristics and major CV event rates of patients with VHR versus non-VHR ASCVD in a real-world setting in the United States (US), hypothesizing that patients with VHR ASCVD would have higher CV event rates.
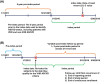
Methods: This was a retrospective cohort study conducted from January 01, 2011, to June 30, 2018, in the US using the Prognos LDL-C database linked to the IQVIA PharMetrics Plus® database supplemented with the IQVIA prescription claims (Dx/LRx) databases. Patients were ≥18 years old and had ≥2 non-ancillary medical claims in the linked databases at least 30 days apart. The study was conducted in 2 stages: (1) identification of patients with ASCVD who met the definition of VHR ASCVD and a matched cohort of non-VHR ASCVD patients using the incidence density sampling (IDS) approach; (2) estimation of the occurrence of major CV events.
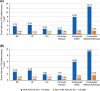
Results: Among patients with ≥1 major ASCVD event (N=147,679), most qualified as VHR ASCVD (79.5%). There were 115,460 patients each in IDS-matched VHR and non-VHR ASCVD cohorts. The composite myocardial infarction/ischemic stroke event rates in the VHR and non-VHR ASCVD cohorts were 8.04 (95% confidence interval [95% CI]: 7.87-8.22) and 0.82 (95% CI: 0.77-0.88) events per 100 patient-years, respectively, during the 1-year post-index period.
Conclusions: Most patients with ≥1 previous major ASCVD event treated in real-world US clinical practice qualified as VHR ASCVD. Patients with VHR ASCVD had much higher rates of major CV events versus non-VHR ASCVD patients.
Keywords: atherosclerotic cardiovascular disease; major cardiovascular events; real-world evidence; very high-risk.
© 2021 The Authors. Clinical Cardiology published by Wiley Periodicals LLC.
Conflict of interest statement
GCF reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Janssen, Merck, and Novartis. MNK is a consultant for Vifor Pharma and reports personal fees from AstraZeneca; grants, personal fees, and other from AstraZeneca; grants and personal fees from Boehringer Ingelheim; and personal fees from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes), Janssen, Bayer, GlaxoSmithKline, Glytec, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. PBR, GV, MH, JA, and KEM were employees and stockholders of Amgen at the time of the study. SN, KS, and RLW were employees of IQVIA at the time of the study, which received consulting fees from Amgen to conduct the study.
Figures
References
-
- Ference BA, Ginsberg HN, Graham I, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459‐2472. - PMC - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. - PubMed
-
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097‐2107. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

