Ninety-Day Follow-up Is Inadequate for Diagnosis of Fracture-related Infections in Patients with Open Fractures
- PMID: 34351311
- PMCID: PMC8673965
- DOI: 10.1097/CORR.0000000000001911
Ninety-Day Follow-up Is Inadequate for Diagnosis of Fracture-related Infections in Patients with Open Fractures
Abstract
Background: Fracture-related infection (FRI) is a challenging complication in musculoskeletal trauma surgery and often complicates the management of open fractures. The CDC currently advocates a surveillance period of 90 days after fracture fixation, but it is unclear what duration of follow-up constitutes adequate surveillance for FRI. Inadequate follow-up will underestimate infections and, in clinical research, will make any interventions studied appear better than they really are, thereby resulting in misleading conclusions.
Questions/purposes: (1) What is the timing of FRI onset in patients with open fractures? (2) What is the proportion of FRIs captured when follow-up is limited to 90 days postoperatively versus when follow-up is extended to 1 year?
Methods: This is a secondary analysis of patient data from a previous retrospective cohort study that investigated whether the duration of perioperative antibiotic prophylaxis was independently associated with FRI in patients with open fractures. Of the 530 eligible patients in the source study, 3% (14) died. Of the remaining 516 patients, 97% (502) patients with 559 long-bone open fractures had 2 years of follow-up constituted the base cohort. Forty-seven fractures in 46 patients were complicated by FRI and were the focus of this secondary analysis. Medical records were reviewed in detail specifically for the current study. Seventy-eight percent (36 of 46) of patients were male, and the mean ± SD age was 42 ± 16 years. The most common mechanism of injury was a motor vehicle accident (63% [29 of 46] of patients), and the tibia was the most involved site (53% [25 of 47] of fractures). The median (interquartile range) time to debridement was 3.0 hours (IQR 2.0 to 4.0). FRIs developed in 3% (7 of 247) of Type I open fractures, 7% (11 of 164) of Type II, 17% (18 of 107) of Type IIIA, 29% (9 of 31) of Type IIIB, and 20% (2 of 10) of Type IIIC open fractures. Each clinic visit of each patient was reviewed, and data about the time of onset of any symptoms and signs suggesting or confirming an FRI, as reported by patients and/or determined by treating surgeons, were recorded. The proportions of FRIs with onset by specific time periods were determined. A Kaplan-Meier survival analysis was performed, and the FRI event rates with 95% confidence intervals were calculated.
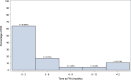
Results: The median (IQR) time to the onset of FRI was 52 days (IQR 15 to 153). Follow-up of 90 days captured only 64% (30 of 47) of FRIs, whereas follow-up of 1 year captured 89% (42 of 47) of FRIs. The proportion of FRIs with onset within 1 year increased to 95% (42 of 44) in the presence of an already healed fracture.
Conclusion: Follow-up of 90 days after the management of an open long-bone fracture is inadequate for postoperative surveillance, especially for research purposes. Clinical research on interventions would report results appearing to be much better than they really are, potentially resulting in misleading conclusions. Follow-up of 1 year is preferable because most FRIs will develop before that time, especially when fracture union has occurred. A small percentage of patients may still develop infections beyond the first year after the management of an open fracture. The risk of missing these infections by not extending follow-up beyond 1 year must be balanced against the additional logistical burden. Future prospective multicenter studies and registries with long-term patient follow-up would help clarify this issue.Level of Evidence Level III, diagnostic study.
Copyright © 2021 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures
Comment in
-
3D-printed Handheld Models Do Not Improve Recognition of Specific Characteristics and Patterns of Three-part and Four-part Proximal Humerus Fractures.Clin Orthop Relat Res. 2022 Jan 1;480(1):150-159. doi: 10.1097/CORR.0000000000001921. Clin Orthop Relat Res. 2022. PMID: 34427569 Free PMC article.
-
CORR Insights®: Ninety-Day Follow-up Is Inadequate for Diagnosis of Fracture-related Infections in Patients with Open Fractures.Clin Orthop Relat Res. 2022 Jan 1;480(1):147-149. doi: 10.1097/CORR.0000000000001964. Clin Orthop Relat Res. 2022. PMID: 34463659 Free PMC article. No abstract available.
References
-
- Amorosa LF, Buirs LD, Bexkens R, et al. A single-stage treatment protocol for presumptive aseptic diaphyseal nonunions: a review of outcomes. J Orthop Trauma. 2013;27:582-586. - PubMed
-
- Centers for Disease Control and Prevention. National Healthcare Safety Network. The National Healthcare Safety Network manual, patient safety component. Available at: https://www.cdc.gov/nhsn/PDFs/pscManual/validation/2013-PSC-Manual-valid.... Accessed February 6, 2020.
-
- Centers for Disease Control and Prevention. Highlighted NHSN January 2013 Patient Safety Component (PSC) manual updates. Available at: https://www.cdc.gov/nhsn/PDFs/Newsletters/January-2013-PSC-Updates.pdf. Accessed February 6, 2020.
-
- Centers for Disease Control and Prevention, National Healthcare Safety Network. Surveillance for surgical site infection events. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Accessed February 6, 2020.
-
- Clinical Orthopaedics and Related Research. Author instructions for submitting to CORR®, clinical research document. Available at: https://journals.lww.com/clinorthop/pages/author-instructions.aspx. Accessed February 6, 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical