Walsogynes H-O from Walsura chrysogyne
- PMID: 34351584
- PMCID: PMC8341256
- DOI: 10.1007/s11418-021-01556-4
Walsogynes H-O from Walsura chrysogyne
Abstract
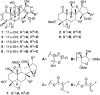
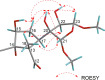
Eight new limonoids, walsogynes H-O (1-8) were isolated from the barks of Walsura chrysogyne, and their structures were determined on the basis of the 1D and 2D NMR data. Walsogynes H-M (1-6) and O (8) were concluded to be 11,12-seco limonoids with a dodecahydro-1H-naphtho[1,8-bc:3,4-c']difuran skeleton, and walsogyne N (7) to be 11,12-seco limonoid sharing a unique dodecahydronaphtho[1,8-bc:5,4-b'c']difuran skeleton. Walsogynes H-O (1-8) exhibited potent antimalarial activity against Plasmodium falciparum 3D7 strain with IC50 value of 2.5, 2.6, 1.6, 2.5, 1.5, 2.6, 2.1, and 1.1 µM, respectively.
Keywords: Antimalarial activity; Limonoids; Walsura chrysogyne.
© 2021. The Japanese Society of Pharmacognosy.
Figures
References
-
- Mabberley DJ. Meliaceae. In: Kubitzki K, editor. Flowering plants Eudicots: Sapindales, Cucurbitales, Myrtaceae. Springer; 2011. pp. 185–211.
-
- Mohamad K, Hirasawa Y, Lim CS, Awang K, Hadi AHA, Takeya K, Morita H. Ceramicine A and walsogyne A, novel limonoids from two species of Meliaceae. Tetrahedron Lett. 2008;49:4276–4278. doi: 10.1016/j.tetlet.2008.04.145. - DOI
-
- Nugroho AE, Okuda M, Yamamoto Y, Wong CP, Hirasawa Y, Kaneda T, Shirota O, Hadi AHA, Morita H. Apowalsogynes A and B, two highly oxidized 3,4-seco-apotirucallane triterpenoids from Walsura chrysogyne. Nat Prod Commun. 2017;12:1189–1192.
-
- Nugroho AE, Okuda M, Yamamoto Y, Hirasawa Y, Wong C-P, Kaneda T, Shirota O, Hadi AHA, Morita H. Walsogynes B–G, limonoids from Walsura chrysogyne. Tetrahedron. 2013;69:4139–4145. doi: 10.1016/j.tet.2013.02.095. - DOI
-
- Sichaem J, Siripong P, Tip-pyang S, Phaopongthai J. A new cytotoxic tirucallane from the twigs of Walsura trichostemon. Nat Prod Commun. 2014;9:367–368. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources