Pancreas cancer and BRCA: A critical subset of patients with improving therapeutic outcomes
- PMID: 34351646
- PMCID: PMC9301324
- DOI: 10.1002/cncr.33812
Pancreas cancer and BRCA: A critical subset of patients with improving therapeutic outcomes
Abstract
Background: Patients with germline/somatic BRCA1/BRCA2 mutations (g/sBRCA1/2) comprise a distinct biologic subgroup of pancreas ductal adenocarcinoma (PDAC).
Methods: Institutional databases were queried to identify patients who had PDAC with g/sBRCA1/2. Demographics, clinicopathologic details, genomic data (annotation sBRCA1/2 according to a precision oncology knowledge base for somatic mutations), zygosity, and outcomes were abstracted. Overall survival (OS) was estimated using the Kaplan-Meier method.
Results: In total, 136 patients with g/sBRCA1/2 were identified between January 2011 and June 2020. Germline BRCA1/2 (gBRCA1/2) mutation was identified in 116 patients (85%). Oncogenic somatic BRCA1/2 (sBRCA1/2) mutation was present in 20 patients (15%). Seventy-seven patients had biallelic BRCA1/2 mutations (83%), and 16 (17%) had heterozygous mutations. Sixty-five patients with stage IV disease received frontline platinum therapy, and 52 (80%) had a partial response. The median OS for entire cohort was 27.6 months (95% CI, 24.9-34.5 months), and the median OS for patients who had stage IV disease was 23 months (95% CI, 19-26 months). Seventy-one patients received a poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor (PARPi), and 52 received PARPi monotherapy. For maintenance PARPi, 10 patients (36%) had a partial response, 12 (43%) had stable disease, and 6 (21%) had progression of disease as their best response. Six patients (21%) received maintenance PARPi for >2 years. For those with stage IV disease who received frontline platinum, the median OS was 26 months (95% CI, 20-52 months) for biallelic patients (n = 39) and 8.66 months (95% CI, 6.2 months to not reached) for heterozygous patients (n = 4). The median OS for those who received PARPi therapy was 26.5 months (95% CI, 24-53 months) for biallelic patients (n = 25) and 8.66 months (95% CI, 7.23 months to not reached) for heterozygous patients (n = 2).
Conclusions: g/sBRCA1/2 mutations did not appear to have different actionable utility. Platinum and PARPi therapies offer therapeutic benefit, and very durable outcomes are observed in a subset of patients who have g/sBRCA1/2 mutations with biallelic status.
Keywords: BRCA; biallelic; germline; pancreas; platinum; somatic; zygosity.
© 2021 American Cancer Society.
Figures


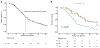
Similar articles
-
Olaparib Use in Patients With Metastatic Breast Cancer Harboring Somatic BRCA1/2 Mutations or Mutations in Non-BRCA1/2, DNA Damage Repair Genes.Clin Breast Cancer. 2022 Jun;22(4):319-325. doi: 10.1016/j.clbc.2021.12.007. Epub 2021 Dec 30. Clin Breast Cancer. 2022. PMID: 35074264
-
Somatic BRCA1/2 mutations are associated with a similar survival advantage to their germline counterparts in tubo-ovarian high grade serous carcinoma.Eur J Cancer. 2025 Mar 26;219:115299. doi: 10.1016/j.ejca.2025.115299. Epub 2025 Feb 11. Eur J Cancer. 2025. PMID: 39955805
-
Chemotherapy and PARP inhibitors in heavily pretreated BRCA1/2 mutation ovarian cancer (BMOC) patients.Gynecol Oncol. 2019 Feb;152(2):270-277. doi: 10.1016/j.ygyno.2018.11.036. Epub 2018 Dec 12. Gynecol Oncol. 2019. PMID: 30551885
-
The role of PARP inhibitors in germline BRCA-associated pancreatic ductal adenocarcinoma.Clin Adv Hematol Oncol. 2020 Mar;18(3):168-179. Clin Adv Hematol Oncol. 2020. PMID: 32609666 Review.
-
Similar response rates and survival with PARP inhibitors for patients with solid tumors harboring somatic versus Germline BRCA mutations: a Meta-analysis and systematic review.BMC Cancer. 2020 Jun 3;20(1):507. doi: 10.1186/s12885-020-06948-5. BMC Cancer. 2020. PMID: 32493233 Free PMC article.
Cited by
-
Clinical Evaluation of the Pancreatic Cancer Microenvironment: Opportunities and Challenges.Cancers (Basel). 2024 Feb 15;16(4):794. doi: 10.3390/cancers16040794. Cancers (Basel). 2024. PMID: 38398185 Free PMC article. Review.
-
Genomic Biomarkers Associated with Response to Induction Chemotherapy in Patients with Localized Pancreatic Ductal Adenocarcinoma.Clin Cancer Res. 2023 Apr 3;29(7):1368-1374. doi: 10.1158/1078-0432.CCR-22-3089. Clin Cancer Res. 2023. PMID: 36795432 Free PMC article.
-
The Landscape and Therapeutic Targeting of BRCA1, BRCA2 and Other DNA Damage Response Genes in Pancreatic Cancer.Curr Issues Mol Biol. 2023 Mar 3;45(3):2105-2120. doi: 10.3390/cimb45030135. Curr Issues Mol Biol. 2023. PMID: 36975505 Free PMC article. Review.
-
Mutational profiling of 103 unresectable pancreatic ductal adenocarcinomas using EUS-guided fine-needle biopsy.Endosc Ultrasound. 2024 May-Jun;13(3):154-164. doi: 10.1097/eus.0000000000000072. Epub 2024 Jul 3. Endosc Ultrasound. 2024. PMID: 39318643 Free PMC article.
-
Pancreatic Cancer: BRCA Targeted Therapy and Beyond.Cancers (Basel). 2023 May 28;15(11):2955. doi: 10.3390/cancers15112955. Cancers (Basel). 2023. PMID: 37296917 Free PMC article. Review.
References
-
- Lowery MA, Jordan EJ, Basturk O, et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: potential actionability and correlation with clinical phenotype. Clin Cancer Res. 2017;23:6094–6100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

