Intravital fluorescence microscopy with negative contrast
- PMID: 34351959
- PMCID: PMC8341626
- DOI: 10.1371/journal.pone.0255204
Intravital fluorescence microscopy with negative contrast
Abstract
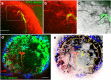
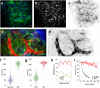
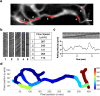
Advances in intravital microscopy (IVM) have enabled the studies of cellular organization and dynamics in the native microenvironment of intact organisms with minimal perturbation. The abilities to track specific cell populations and monitor their interactions have opened up new horizons for visualizing cell biology in vivo, yet the success of standard fluorescence cell labeling approaches for IVM comes with a "dark side" in that unlabeled cells are invisible, leaving labeled cells or structures to appear isolated in space, devoid of their surroundings and lacking proper biological context. Here we describe a novel method for "filling in the void" by harnessing the ubiquity of extracellular (interstitial) fluid and its ease of fluorescence labelling by commonly used vascular and lymphatic tracers. We show that during routine labeling of the vasculature and lymphatics for IVM, commonly used fluorescent tracers readily perfuse the interstitial spaces of the bone marrow (BM) and the lymph node (LN), outlining the unlabeled cells and forming negative contrast images that complement standard (positive) cell labeling approaches. The method is simple yet powerful, offering a comprehensive view of the cellular landscape such as cell density and spatial distribution, as well as dynamic processes such as cell motility and transmigration across the vascular endothelium. The extracellular localization of the dye and the interstitial flow provide favorable conditions for prolonged Intravital time lapse imaging with minimal toxicity and photobleaching.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures