Dose-dependent Respiratory Depression by Remifentanil in the Rabbit Parabrachial Nucleus/Kölliker-Fuse Complex and Pre-Bötzinger Complex
- PMID: 34352068
- PMCID: PMC8489159
- DOI: 10.1097/ALN.0000000000003886
Dose-dependent Respiratory Depression by Remifentanil in the Rabbit Parabrachial Nucleus/Kölliker-Fuse Complex and Pre-Bötzinger Complex
Abstract
Background: Recent studies showed partial reversal of opioid-induced respiratory depression in the pre-Bötzinger complex and the parabrachial nucleus/Kölliker-Fuse complex. The hypothesis for this study was that opioid antagonism in the parabrachial nucleus/Kölliker-Fuse complex plus pre-Bötzinger complex completely reverses respiratory depression from clinically relevant opioid concentrations.
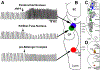
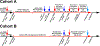
Methods: Experiments were performed in 48 adult, artificially ventilated, decerebrate rabbits. The authors decreased baseline respiratory rate ~50% with intravenous, "analgesic" remifentanil infusion or produced apnea with remifentanil boluses and investigated the reversal with naloxone microinjections (1 mM, 700 nl) into the Kölliker-Fuse nucleus, parabrachial nucleus, and pre-Bötzinger complex. In another group of animals, naloxone was injected only into the pre-Bötzinger complex to determine whether prior parabrachial nucleus/Kölliker-Fuse complex injection impacted the naloxone effect. Last, the µ-opioid receptor agonist [d-Ala,2N-MePhe,4Gly-ol]-enkephalin (100 μM, 700 nl) was injected into the parabrachial nucleus/Kölliker-Fuse complex. The data are presented as medians (25 to 75%).
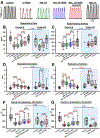
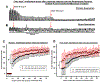
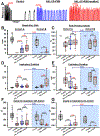
Results: Remifentanil infusion reduced the respiratory rate from 36 (31 to 40) to 16 (15 to 21) breaths/min. Naloxone microinjections into the bilateral Kölliker-Fuse nucleus, parabrachial nucleus, and pre-Bötzinger complex increased the rate to 17 (16 to 22, n = 19, P = 0.005), 23 (19 to 29, n = 19, P < 0.001), and 25 (22 to 28) breaths/min (n = 11, P < 0.001), respectively. Naloxone injection into the parabrachial nucleus/Kölliker-Fuse complex prevented apnea in 12 of 17 animals, increasing the respiratory rate to 10 (0 to 12) breaths/min (P < 0.001); subsequent pre-Bötzinger complex injection prevented apnea in all animals (13 [10 to 19] breaths/min, n = 12, P = 0.002). Naloxone injection into the pre-Bötzinger complex alone increased the respiratory rate to 21 (15 to 26) breaths/min during analgesic concentrations (n = 10, P = 0.008) but not during apnea (0 [0 to 0] breaths/min, n = 9, P = 0.500). [d-Ala,2N-MePhe,4Gly-ol]-enkephalin injection into the parabrachial nucleus/Kölliker-Fuse complex decreased respiratory rate to 3 (2 to 6) breaths/min.
Conclusions: Opioid reversal in the parabrachial nucleus/Kölliker-Fuse complex plus pre-Bötzinger complex only partially reversed respiratory depression from analgesic and even less from "apneic" opioid doses. The lack of recovery pointed to opioid-induced depression of respiratory drive that determines the activity of these areas.
Copyright © 2021, the American Society of Anesthesiologists. All Rights Reserved.
Conflict of interest statement
Figures


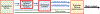
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

