A simple model explains the cell cycle-dependent assembly of centromeric nucleosomes in holocentric species
- PMID: 34352103
- PMCID: PMC8450114
- DOI: 10.1093/nar/gkab648
A simple model explains the cell cycle-dependent assembly of centromeric nucleosomes in holocentric species
Abstract
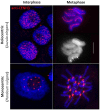
Centromeres are essential for chromosome movement. In independent taxa, species with holocentric chromosomes exist. In contrast to monocentric species, where no obvious dispersion of centromeres occurs during interphase, the organization of holocentromeres differs between condensed and decondensed chromosomes. During interphase, centromeres are dispersed into a large number of CENH3-positive nucleosome clusters in a number of holocentric species. With the onset of chromosome condensation, the centromeric nucleosomes join and form line-like holocentromeres. Using polymer simulations, we propose a mechanism relying on the interaction between centromeric nucleosomes and structural maintenance of chromosomes (SMC) proteins. Different sets of molecular dynamic simulations were evaluated by testing four parameters: (i) the concentration of Loop Extruders (LEs) corresponding to SMCs, (ii) the distribution and number of centromeric nucleosomes, (iii) the effect of centromeric nucleosomes on interacting LEs and (iv) the assembly of kinetochores bound to centromeric nucleosomes. We observed the formation of a line-like holocentromere, due to the aggregation of the centromeric nucleosomes when the chromosome was compacted into loops. A groove-like holocentromere structure formed after a kinetochore complex was simulated along the centromeric line. Similar mechanisms may also organize a monocentric chromosome constriction, and its regulation may cause different centromere types during evolution.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
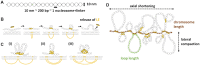




Similar articles
-
Holocentromeres are dispersed point centromeres localized at transcription factor hotspots.Elife. 2014 Jan 1;3:e02025. doi: 10.7554/eLife.02025. Elife. 2014. PMID: 24714495 Free PMC article.
-
Structure, dynamics, and evolution of centromeric nucleosomes.Proc Natl Acad Sci U S A. 2007 Oct 9;104(41):15974-81. doi: 10.1073/pnas.0707648104. Epub 2007 Sep 24. Proc Natl Acad Sci U S A. 2007. PMID: 17893333 Free PMC article. Review.
-
Diversity in the organization of centromeric chromatin.Curr Opin Genet Dev. 2015 Apr;31:28-35. doi: 10.1016/j.gde.2015.03.010. Epub 2015 May 16. Curr Opin Genet Dev. 2015. PMID: 25956076 Review.
-
Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells.PLoS Biol. 2007 Aug;5(8):e218. doi: 10.1371/journal.pbio.0050218. PLoS Biol. 2007. PMID: 17676993 Free PMC article.
-
Centromere identity is specified by a single centromeric nucleosome in budding yeast.Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14706-11. doi: 10.1073/pnas.0706985104. Epub 2007 Sep 5. Proc Natl Acad Sci U S A. 2007. PMID: 17804787 Free PMC article.
Cited by
-
Holocentromeres can consist of merely a few megabase-sized satellite arrays.Nat Commun. 2023 Jun 13;14(1):3502. doi: 10.1038/s41467-023-38922-7. Nat Commun. 2023. PMID: 37311740 Free PMC article.
-
Plasticity in centromere organization and kinetochore composition: Lessons from diversity.Curr Opin Cell Biol. 2022 Feb;74:47-54. doi: 10.1016/j.ceb.2021.12.007. Epub 2022 Feb 2. Curr Opin Cell Biol. 2022. PMID: 35108654 Free PMC article. Review.
-
Consistencies and contradictions in different polymer models of chromatin architecture.Comput Struct Biotechnol J. 2023 Jan 24;21:1084-1091. doi: 10.1016/j.csbj.2023.01.033. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36789261 Free PMC article. Review.
-
Understanding and Simulating the Dynamics of a Polymer-Like Chromatin.Methods Mol Biol. 2025;2873:283-302. doi: 10.1007/978-1-0716-4228-3_16. Methods Mol Biol. 2025. PMID: 39576608 Review.
-
Super-resolution microscopy reveals the number and distribution of topoisomerase IIα and CENH3 molecules within barley metaphase chromosomes.Chromosoma. 2023 Mar;132(1):19-29. doi: 10.1007/s00412-023-00785-8. Epub 2023 Jan 31. Chromosoma. 2023. PMID: 36719450 Free PMC article.
References
-
- Melters D.P., Paliulis L.V., Korf I.F., Chan S.W.L.. Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res. 2012; 20:579–593. - PubMed
-
- Escudero M., Mrquez-Corro J.I., Hipp A.L.. The phylogenetic origins and evolutionary history of holocentric chromosomes. Syst. Bot. 2016; 41:580–585.
-
- Král J., Forman M., Kořínková T., Lerma A.C.R., Haddad C.R., Musilová J., Řezáč M., Herrera I.M.Á., Thakur S., Dippenaar-Schoeman A.S.et al. .. Insights into the karyotype and genome evolution of haplogyne spiders indicate a polyploid origin of lineage with holokinetic chromosomes. Sci. Rep.-uk. 2019; 9:3001. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

