The 5-formyl-tetrahydrofolate proteome links folates with C/N metabolism and reveals feedback regulation of folate biosynthesis
- PMID: 34352110
- PMCID: PMC8505879
- DOI: 10.1093/plcell/koab198
The 5-formyl-tetrahydrofolate proteome links folates with C/N metabolism and reveals feedback regulation of folate biosynthesis
Abstract
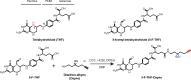
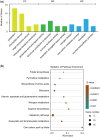
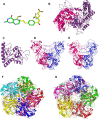
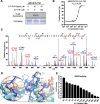
Folates are indispensable for plant development, but their molecular mode of action remains elusive. We synthesized a probe, "5-F-THF-Dayne," comprising 5-formyl-tetrahydrofolate (THF) coupled to a photoaffinity tag. Exploiting this probe in an affinity proteomics study in Arabidopsis thaliana, we retrieved 51 hits. Thirty interactions were independently validated with in vitro expressed proteins to bind 5-F-THF with high or low affinity. Interestingly, the interactors reveal associations beyond one-carbon metabolism, covering also connections to nitrogen (N) metabolism, carbohydrate metabolism/photosynthesis, and proteostasis. Two of the interactions, one with the folate biosynthetic enzyme DIHYDROFOLATE REDUCTASE-THYMIDYLATE SYNTHASE 1 (AtDHFR-TS1) and another with N metabolism-associated glutamine synthetase 1;4 (AtGLN1;4), were further characterized. In silico and experimental analyses revealed G35/K36 and E330 as key residues for the binding of 5-F-THF in AtDHFR-TS1 and AtGLN1;4, respectively. Site-directed mutagenesis of AtGLN1;4 E330, which co-localizes with the ATP-binding pocket, abolished 5-F-THF binding as well as AtGLN1;4 activity. Furthermore, 5-F-THF was noted to competitively inhibit the activities of AtDHFR-TS1 and AtGLN1;4. In summary, we demonstrated a regulatory role for 5-F-THF in N metabolism, revealed 5-F-THF-mediated feedback regulation of folate biosynthesis, and identified a total of 14 previously unknown high-affinity binding cellular targets of 5-F-THF. Together, this sets a landmark toward understanding the role of folates in plant development.
� American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Site-directed mutagenesis of a highly conserved aspartate in the putative 10-formyl-tetrahydrofolate binding site of yeast C1-tetrahydrofolate synthase.Arch Biochem Biophys. 1996 Sep 1;333(1):251-9. doi: 10.1006/abbi.1996.0388. Arch Biochem Biophys. 1996. PMID: 8806778
-
The metabolism of some folates in the rat.Br J Nutr. 1975 May;33(3):299-308. doi: 10.1079/bjn19750035. Br J Nutr. 1975. PMID: 1079138
-
Arabidopsis plastidial folylpolyglutamate synthetase is required for nitrogen metabolism under nitrate-limited condition in darkness.Biochem Biophys Res Commun. 2017 Jan 8;482(2):277-281. doi: 10.1016/j.bbrc.2016.11.054. Epub 2016 Nov 12. Biochem Biophys Res Commun. 2017. PMID: 27845046
-
Challenges in the determination of unsubstituted food folates: impact of stabilities and conversions on analytical results.J Agric Food Chem. 2015 Mar 11;63(9):2367-77. doi: 10.1021/jf504987n. Epub 2015 Feb 26. J Agric Food Chem. 2015. PMID: 25642846 Review.
-
Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics.Clin Pharmacokinet. 2010 Aug;49(8):535-48. doi: 10.2165/11532990-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20608755 Review.
Cited by
-
Folate from probiotic bacteria and its therapeutic applications.Arch Microbiol. 2025 Apr 18;207(6):124. doi: 10.1007/s00203-025-04327-x. Arch Microbiol. 2025. PMID: 40249393 Review.
-
B vitamin supply in plants and humans: the importance of vitamer homeostasis.Plant J. 2022 Aug;111(3):662-682. doi: 10.1111/tpj.15859. Epub 2022 Jun 27. Plant J. 2022. PMID: 35673947 Free PMC article. Review.
-
Reduced γ-glutamyl hydrolase activity likely contributes to high folate levels in Periyakulam-1 tomato.Hortic Res. 2022 Nov 19;10(1):uhac235. doi: 10.1093/hr/uhac235. eCollection 2023. Hortic Res. 2022. PMID: 36643736 Free PMC article.
-
Folate depletion impact on the cell cycle results in restricted primary root growth in Arabidopsis.Plant Mol Biol. 2025 Feb 13;115(2):31. doi: 10.1007/s11103-025-01554-0. Plant Mol Biol. 2025. PMID: 39946030 Free PMC article.
-
The serine-glycine-one-carbon metabolic network orchestrates changes in nitrogen and sulfur metabolism and shapes plant development.Plant Cell. 2024 Jan 30;36(2):404-426. doi: 10.1093/plcell/koad256. Plant Cell. 2024. PMID: 37804096 Free PMC article.
References
-
- Blancquaert D, De Steur H, Gellynck X, Van Der Straeten D (2014) Present and future of folate biofortification of crop plants. J Exp Bot 65: 895–906 - PubMed
-
- Blancquaert D, Van Daele J, Strobbe S, Kiekens F, Storozhenko S, De Steur H, Gellynck X, Lambert W, Stove C, Van Der Straeten D (2015) Improving folate (vitamin B-9) stability in biofortified rice through metabolic engineering. Nat Biotechnol 33: 1076. - PubMed
-
- Conway LP, Li WC, Parker CG, , (2021) Chemoproteomic-enabled phenotypic screening. Cell Chem Biol 28: 371–393 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

