Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients
- PMID: 34352226
- PMCID: PMC8299183
- DOI: 10.1016/j.celrep.2021.109504
Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients
Abstract
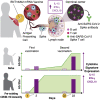
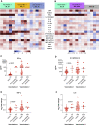
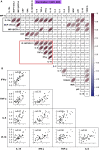
Early responses to vaccination are important for shaping both humoral and cellular protective immunity. Dissecting innate vaccine signatures may predict immunogenicity to help optimize the efficacy of mRNA and other vaccine strategies. Here, we characterize the cytokine and chemokine responses to the 1st and 2nd dose of the BNT162b2 mRNA (Pfizer/BioNtech) vaccine in antigen-naive and in previously coronavirus disease 2019 (COVID-19)-infected individuals (NCT04743388). Transient increases in interleukin-15 (IL-15) and interferon gamma (IFN-γ) levels early after boost correlate with Spike antibody levels, supporting their use as biomarkers of effective humoral immunity development in response to vaccination. We identify a systemic signature including increases in IL-15, IFN-γ, and IP-10/CXCL10 after the 1st vaccination, which were enriched by tumor necrosis factor alpha (TNF-α) and IL-6 after the 2nd vaccination. In previously COVID-19-infected individuals, a single vaccination results in both strong cytokine induction and antibody titers similar to the ones observed upon booster vaccination in antigen-naive individuals, a result with potential implication for future public health recommendations.
Keywords: IFN-γ; IL-15; IP10/CXCL10; SARS-CoV-2; Spike antibody; cytokines; innate immunity; mRNA vaccine.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Andersen-Nissen E., Fiore-Gartland A., Ballweber Fleming L., Carpp L.N., Naidoo A.F., Harper M.S., Voillet V., Grunenberg N., Laher F., Innes C., et al. Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021;17:e1009363. - PMC - PubMed
-
- Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., Halkerston R., Hill J., Jenkin D., Stockdale L., et al. Oxford COVID Vaccine Trial Group Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021;27:279–288. - PubMed
-
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. HGID Lab. NIAID-USUHS Immune Response to COVID Group. COVID Clinicians. COVID-STORM Clinicians. Imagine COVID Group. French COVID Cohort Study Group. Milieu Intérieur Consortium. CoV-Contact Cohort. Amsterdam UMC Covid-19 Biobank. COVID Human Genetic Effort Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

