Interactions of HIV gp41's membrane-proximal external region and transmembrane domain with phospholipid membranes from 31P NMR
- PMID: 34352242
- PMCID: PMC8826538
- DOI: 10.1016/j.bbamem.2021.183723
Interactions of HIV gp41's membrane-proximal external region and transmembrane domain with phospholipid membranes from 31P NMR
Abstract
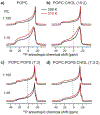
HIV-1 entry into cells requires coordinated changes of the conformation and dynamics of both the fusion protein, gp41, and the lipids in the cell membrane and virus envelope. Commonly proposed features of membrane deformation during fusion include high membrane curvature, lipid disorder, and membrane surface dehydration. The virus envelope and target cell membrane contain a diverse set of phospholipids and cholesterol. To dissect how different lipids interact with gp41 to contribute to membrane fusion, here we use 31P solid-state NMR spectroscopy to investigate the curvature, dynamics, and hydration of POPE, POPC and POPS membranes, with and without cholesterol, in the presence of a peptide comprising the membrane proximal external region (MPER) and transmembrane domain (TMD) of gp41. Static 31P NMR spectra indicate that the MPER-TMD induces strong negative Gaussian curvature (NGC) to the POPE membrane but little curvature to POPC and POPC:POPS membranes. The NGC manifests as an isotropic peak in the static NMR spectra, whose intensity increases with the peptide concentration. Cholesterol inhibits the NGC formation and stabilizes the lamellar phase. Relative intensities of magic-angle spinning 31P cross-polarization and direct-polarization spectra indicate that all three phospholipids become more mobile upon peptide binding. Finally, 2D 1H-31P correlation spectra show that the MPER-TMD enhances water 1H polarization transfer to the lipids, indicating that the membrane surfaces become more hydrated. These results suggest that POPE is an essential component of the high-curvature fusion site, and lipid dynamic disorder is a general feature of membrane restructuring during fusion.
Keywords: Cholesterol; Membrane curvature; Protein dynamics; Virus-cell fusion.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures
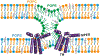


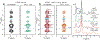



References
-
- Weissman D, Rabin RL, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber JM, Fauci AS, Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor, Nature, 389 981–985 (1997). - PubMed
-
- Chernomordik LV, Kozlov MM, Protein-lipid interplay in fusion and fission of biological membranes, Annual review of biochemistry, 72 175–207 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

