Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019
- PMID: 34352418
- PMCID: PMC8327605
- DOI: 10.1016/j.jvsv.2021.07.009
Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019
Abstract
Since December 2020, four vaccines for SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) have been developed, and three have been approved for immediate use in the United States. Two are mRNA vaccines, and one uses a viral vector mechanism. Thrombotic complications have been reported after vaccine administration, which were primarily cerebral sinus thromboses after administration of the viral vector vaccines. To the best of our knowledge, we are the first to report venous thrombotic complications within days of administration of the mRNA-1273 (Moderna) vaccine. We present a series of three women who developed venous thromboembolism after RNA-1273 vaccination at a single healthcare system.
Keywords: COVID-19; Deep vein thrombosis; Pulmonary embolism; Venous thromboembolism.
Copyright © 2021 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Figures
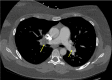
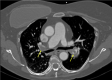
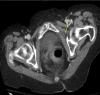
Comment in
-
Reply.J Vasc Surg Venous Lymphat Disord. 2022 Jan;10(1):285. doi: 10.1016/j.jvsv.2021.09.008. J Vasc Surg Venous Lymphat Disord. 2022. PMID: 34920849 Free PMC article. No abstract available.
-
Acute venous thromboembolism after COVID-19 vaccination.J Vasc Surg Venous Lymphat Disord. 2022 Jan;10(1):285. doi: 10.1016/j.jvsv.2021.08.024. J Vasc Surg Venous Lymphat Disord. 2022. PMID: 34920850 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html Available at: - PubMed
-
- Marks P., Schuchat A. Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-st... Available at:
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

