Repetitive Low-Level Blast Exposure Improves Behavioral Deficits and Chronically Lowers Aβ42 in an Alzheimer Disease Transgenic Mouse Model
- PMID: 34353119
- PMCID: PMC8820291
- DOI: 10.1089/neu.2021.0184
Repetitive Low-Level Blast Exposure Improves Behavioral Deficits and Chronically Lowers Aβ42 in an Alzheimer Disease Transgenic Mouse Model
Abstract
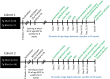
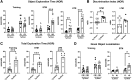
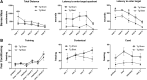
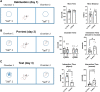
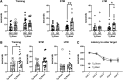
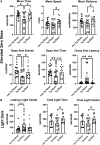
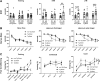
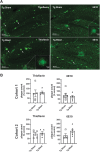
Public awareness of traumatic brain injury (TBI) in the military increased recently because of the conflicts in Iraq and Afghanistan where blast injury was the most common mechanism of injury. Besides overt injuries, concerns also exist over the potential adverse consequences of subclinical blast exposures, which are common for many service members. A TBI is a risk factor for the later development of neurodegenerative diseases, including Alzheimer disease (AD)-like disorders. Studies of acute TBI in humans and animals have suggested that increased processing of the amyloid precursor protein (APP) toward the amyloid beta protein (Aβ) may explain the epidemiological associations with AD. In a previous study, however, we found in both rat and mouse models of blast overpressure exposure that rather than increasing, rodent brain Aβ42 levels were decreased after acute blast exposure. Here we subjected APP/presenilin 1 transgenic mice (APP/PS1 Tg) to an extended sequence of repetitive low-level blast exposures (34.5 kPa) administered three times per week over eight weeks. If initiated at 20 weeks of age, these repetitive exposures, which were designed to mimic human subclinical blast exposures, reduced anxiety and improved cognition as well as social interactions in APP/PS1 Tg mice, returning many behavioral parameters in APP/PS1 Tg mice to levels of non-transgenic wild type mice. Repetitive low-level blast exposure was less effective at improving behavioral deficits in APP/PS1 Tg mice when begun at 36 weeks of age. While amyloid plaque loads were unchanged, Aβ 42 levels and Aβ oligomers were reduced in the brain of mice exposed to repetitive low-level blast exposures initiated at 20 weeks of age, although levels did not directly correlate with behavioral parameters in individual animals. These results have implications for understanding the nature of blast effects on the brain and their relationship to human neurodegenerative diseases.
Keywords: Alzheimer disease; amyloid beta protein; blast; transgenic mouse; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures








Similar articles
-
Blast-Mediated Traumatic Brain Injury Exacerbates Retinal Damage and Amyloidosis in the APPswePSENd19e Mouse Model of Alzheimer's Disease.Invest Ophthalmol Vis Sci. 2019 Jun 3;60(7):2716-2725. doi: 10.1167/iovs.18-26353. Invest Ophthalmol Vis Sci. 2019. PMID: 31247112 Free PMC article.
-
Chronic effects of blast injury on the microvasculature in a transgenic mouse model of Alzheimer's disease related Aβ amyloidosis.Fluids Barriers CNS. 2022 Jan 10;19(1):5. doi: 10.1186/s12987-021-00301-z. Fluids Barriers CNS. 2022. PMID: 35012589 Free PMC article.
-
Expression of the plant viral protease NIa in the brain of a mouse model of Alzheimer's disease mitigates Aβ pathology and improves cognitive function.Exp Mol Med. 2012 Dec 31;44(12):740-8. doi: 10.3858/emm.2012.44.12.082. Exp Mol Med. 2012. PMID: 23172351 Free PMC article.
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
-
Effects of low-level blast exposure on the nervous system: is there really a controversy?Front Neurol. 2014 Dec 19;5:269. doi: 10.3389/fneur.2014.00269. eCollection 2014. Front Neurol. 2014. PMID: 25566175 Free PMC article. Review.
Cited by
-
Update on modifiable risk factors for Alzheimer's disease and related dementias.Curr Opin Neurol. 2024 Apr 1;37(2):166-181. doi: 10.1097/WCO.0000000000001243. Epub 2024 Jan 24. Curr Opin Neurol. 2024. PMID: 38265228 Free PMC article. Review.
-
Exploring Calcium Channels as Potential Therapeutic Targets in Blast Traumatic Brain Injury.Pharmaceuticals (Basel). 2025 Feb 6;18(2):223. doi: 10.3390/ph18020223. Pharmaceuticals (Basel). 2025. PMID: 40006037 Free PMC article. Review.
-
Modeling Highly Repetitive Low-level Blast Exposure in Mice.J Vis Exp. 2024 May 24;(207):10.3791/66592. doi: 10.3791/66592. J Vis Exp. 2024. PMID: 38856207 Free PMC article.
-
(2R,6R)-Hydroxynorketamine Treatment of Rats Exposed to Repetitive Low-Level Blast Injury.Neurotrauma Rep. 2023 Mar 30;4(1):197-217. doi: 10.1089/neur.2022.0088. eCollection 2023. Neurotrauma Rep. 2023. PMID: 37020715 Free PMC article.
-
Inflammasome activation in traumatic brain injury and Alzheimer's disease.Transl Res. 2023 Apr;254:1-12. doi: 10.1016/j.trsl.2022.08.014. Epub 2022 Sep 5. Transl Res. 2023. PMID: 36070840 Free PMC article. Review.
References
-
- Elder, G.A., Ehrlich, M.E., and Gandy, S. (2019). Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci. Lett. 707, 134294. - PubMed
-
- Hoge, C.W., McGurk, D., Thomas, J.L., Cox, A.L., Engel, C.C., and Castro, C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463. - PubMed
-
- Engel, C., Hoch, E. and Simmons, M. (2019). The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health. Proceedings, Findings, and Expert Recommendations from the Seventh Department of Defense State-of-the-Science Meeting. Arlington, VA: Rand Corporation.
-
- Carr, W., Stone, J.R., Walilko, T., Young, L.A., Snook, T.L., Paggi, M.E., Tsao, J.W., Jankosky, C.J., Parish, R.V., and Ahlers, S.T. (2016). Repeated low-level blast exposure: a descriptive human subjects study. Mil. Med. 181, Suppl. 5, 28–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

